Abstract
Paliperidone, the major active metabolite of risperidone, is an atypical antipsychotic agent formulated as an extended-release (ER) tablet suitable for once-daily oral administration. Paliperidone ER is approved for the treatment of adolescents aged 12–17 years with schizophrenia in the US (the focus of this review). It is also approved for the treatment of adults with schizophrenia or schizoaffective disorder.
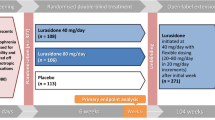
Paliperidone ER has shown efficacy in the treatment of patients aged 12–17 years with acutely symptomatic schizophrenia in a randomized, double-blind, parallel-group, placebo-controlled, multicenter, 6-week trial. The primary endpoint was the change from baseline in Positive and Negative Syndrome Scale (PANSS) total score to day 43 or the final assessment point post-baseline.
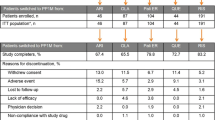
Patients with a PANSS total score of 60–120 received one of three weight-based, fixed once-daily doses of paliperidone ER (patients weighing 29 kg to <51 kg: 1.5mg [low-dose], 3mg [medium], or 6mg [high]; patients weighing >51 kg: 1.5mg [low], 6mg [medium], or 12mg [high]), or placebo.
Compared with placebo, significant improvements in mean PANSS total scores were reported for the medium-dose (3–6mg) paliperidone ER treatment groups. There were no significant differences in mean PANSS total scores between the recipients of low-dose or high-dose paliperidone ER versus placebo. Mean PANSS total scores in the actual dose treatment groups (regardless of weight) decreased from baseline (i.e. improved) and were significantly lower for the 3, 6, and 12mg groups than for the placebo group.
Treatment-emergent adverse events were dose related in adolescents with schizophrenia who received weight-based fixed doses of paliperidone ER.




Similar content being viewed by others
References
Masi G, Liboni F. Management of schizophrenia in children and adolescents: focus on pharmacotherapy. Drugs 2011 Jan 22; 71 (2): 179–208
Gentile S. Clinical usefulness of second-generation antipsychotics in treating children and adolescents diagnosed with bipolar or schizophrenic disorders. Paediatr Drugs 2011 Oct 1; 13 (5): 291–302
Pandina G, Petersen T, Singh J, et al. Cognitive functioning in adolescents with schizsophrenia treated with paliperidone: interim data from a 6-month, open-label study [poster no. 34]. 58th Annual Meeting of the American Academy of Child and Adolescent Psychiatry; 2011 Oct 18–23; Toronto (ON)
Singh J, Robb A, Vijapurkar U, et al. A randomized, double-blind study of paliperidone extended-release in treatment of acute schizophrenia in adolescents. Biol Psychiatry 2011 Dec 15; 70 (12): 1179–87
Masi G, Mucci M, Pari C. Children with schizophrenia: clinical picture and pharmacological treatment. CNS Drugs 2006; 20 (10): 841–66
Janssen Pharmaceuticals, Inc. Invega® (paliperidone) extended-release tablets: US prescribing information [online]. Available from URL: http://www.janssenmedicalinformation.com [Accessed 2012 Oct 2]
Conley R, Gupta SK, Sathyan G. Clinical spectrum of the osmotic-controlled release oral delivery system (OROS), an advanced oral delivery form. Curr Med Res Opin 2006 Oct; 22 (10): 1879–92
Yang LPH, Plosker GL. Paliperidone extended release. CNS Drugs 2007; 21 (5): 417–25; discussion 26–7
Chwieduk CM, Keating GM. Paliperidone extended release: a review of its use in the management of schizophrenia. Drugs 2010 Jul 9; 70 (10): 1295–317
Yang LPH. Oral paliperidone: a review of its use in the management of schizoaffective disorder. CNS Drugs 2011; 25 (6): 525–38
Schwartzer JJ, Morrison RL, Ricci LA, et al. Paliperidone suppresses the development of the aggressive phenotype in a developmentally sensitive animal model ofescalated aggression. Psychopharmacology (Berl) 2009 May; 203 (4): 653–63
Corena-McLeod M del P, Oliveros A, Charlesworth C, et al. Paliperidone as a mood stabilizer: a pre-frontal cortex synaptoneurosomal proteomics comparison with lithium and valproic acid after chronic treatment reveals similarities in protein expression. Brain Res 2008 Oct 3; 1233: 8–19
Muly EC, Votaw JR, Ritchie J, et al. Relationship between dose, drug levels, and D2 receptor occupancy for the atypical antipsychotics risperidone and paliperidone. J Pharmacol Exp Ther 2012 Apr; 341 (1): 81–9
Arakawa R, Ito H, Takano A, et al. Dose-finding study of paliperidone ER based on striatal and extrastriatal dopamine D2 receptor occupancy in patients with schizophrenia. Psychopharmacology (Berl) 2008 Apr; 197 (2): 229–35
Luthringer R, Staner L, Noel N, et al. A double-blind, placebo-controlled, randomized study evaluating the effect of paliperidone extended-release tablets on sleep architecture in patients with schizophrenia. Int Clin Psychopharmacol 2007 Sep; 22 (5): 299–308
Knegtering R, Baselmans P, Castelein S, et al. Predominant role of the 9-hydroxy metabolite of risperidone in elevating blood prolactin levels. Am J Psychiatry 2005 May; 162 (5): 1010–2
Berwaerts J, Cleton A, Rossenu S, et al. A comparison of serum prolactin concentrations after administration of paliperidone extended-release and risperidone tablets in patients with schizophrenia. J Psychopharmacol 2010 Jul; 24 (7): 1011–8
Richelson E. New antipsychotic drugs: how do their receptor-binding profiles compare? J Clin Psychiatry 2010 Sep; 71 (9): 1243–4
Richelson E, Souder T. Binding of antipsychotic drugs to human brain receptors focus on newer generation compounds. Life Sci 2000 Nov 24; 68 (1): 29–39
Kapur S, Seeman P. Does fast dissociation from the dopamine D2 receptor explain the action of atypical antipsychotics?: A new hypothesis. Am J Psych 2001 Mar; 158 (3): 360–9
Rosenbloom AL. Hyperprolactinemia with antipsychotic drugs in children and adolescents. Int J Pediatr Endocrinol. Epub 2010 Aug 24
Boom S, Talluri K, Janssens L, et al. Single- and multiple-dose pharmacokinetics and dose proportionality of the psychotropic agent paliperidone extended release. J Clin Pharmacol 2009 Nov; 49 (11): 1318–30
Vermeir M, Naessens I, Remmerie B, et al. Absorption, metabolism, and excretion of paliperidone, a new monoaminergic antagonist, in humans. Drug Metab and Dispos 2008 Apr; 36 (4): 769–79
de Leon J, Wynn G, Sandson NB. The pharmacokinetics of paliperidone versus risperidone. Psychosomatics 2010 Jan–Feb; 51 (1): 80–8
Canuso CM, Battisti WP. Paliperidone extended-release: a review of efficacy and tolerability in schizophrenia, schizoaffective disorder and bipolar mania. Expert Opin Pharmacother 2010 Oct; 11 (15): 2557–67
Boom S, Thyssen A, Crauwels H, et al. The influence of hepatic impairment on the pharmacokinetics of paliperidone. Int J Clin Pharmacol Ther 2009 Oct; 47 (10): 606–16
Tianmei S, Liang S, Yi L, et al. Single-dose pharmacokinetics of paliperidone extended-release tablets in healthy Chinese subjects. Human Psychopharm Clin 2010; 25: 404–9
Zhu HJ, Wang JS, Markowitz JS, et al. Risperidone and paliperidone inhibit p-glycoprotein activity in vitro. Neuropsychopharmacology 2007 Apr; 32 (4): 757–64
Berwaerts J, Cleton A, Herben V, et al. The effects of paroxetine on the pharmacokinetics of paliperidone extended-release tablets. Pharmacopsychiatry 2009 Jul; 42 (4): 158–63
Thyssen A, Cleton A, Talluri K, et al. No pharmacokinetic interaction between paliperidone extended-release tablets and trimethoprim in healthy subjects. Hum Psychopharmacol 2009 Oct; 24 (7): 532–9
Malaspina D, Mark O, Gopal S, et al. Paternal age and treatment response in adolescents with schizophrenia [abstract 53]. Neuropsychopharmacology 2011 Dec; 36: S99
Davidson M, Emsley R, Kramer M, et al. Efficacy, safety and early response of paliperidone extended-release tablets (paliperidone ER): results of a 6-week, randomized, placebo-controlled study. Schizophr Res 2007 July; 93 (1–3): 117–30
Kane J, Canas F, Kramer M, et al. Treatment of schizophrenia with paliperidone extended-release tablets: a 6-week placebo-controlled trial. Schizophr Res 2007 February; 90 (1–3): 147–61
Marder SR, Kramer M, Ford L, et al. Efficacy and safety of paliperidone extended-release tablets: results of a 6-week, randomized, placebo-controlled Study. Biol Psychiatry 2007 15 Dec; 62 (12): 1363–70
Canuso CM, Dirks B, Carothers J, et al. Randomized, double-blind, placebo-controlled study of paliperidone extended-release and quetiapine in inpatients with recently exacerbated schizophrenia. Am J Psychiatry 2009 Jun; 166 (6): 691–701
Tzimos A, Samokhvalov V, Kramer M, et al. Safety and tolerability of oral paliperidone extended-release tablets in elderly patients with schizophrenia: a double-blind, placebo-controlled study with six-month open-label extension. Am J Geriatr Psychiatry 2008 Jan; 16 (1): 31–43
Rajji TK, Ismail Z, Mulsant BH. Age at onset and cognition in schizophrenia: meta-analysis. Br J Psychiatry 2009 Oct; 195 (4): 286–93
Meltzer HY, Bobo WV, Nuamah IF, et al. Efficacy and tolerability of oral paliperidone extended-release tablets in the treatment of acute schizophrenia: pooled data from three 6-week, placebo-controlled studies. J Clin Psychiatry 2008 May; 69 (5): 817–29
Acknowledgments and Disclosures
The manuscript was reviewed by: M.D. Jibson, Department of Psychiatry, University of Michigan, Ann Arbor, MI, USA; A.S. Robb, Department of Psychiatry and Behavioral Sciences and Pediatrics, Children’s National Medical Center, Washington, DC, USA.
The preparation of this review was not supported by any external funding. During the peer review process, the manufacturer of the agent under review was also offerred an opportunity to comment on this article. Changes resulting from comments received were made by the author on the basis of scientific and editorial merit.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Perry, C.M. Paliperidone Extended Release. Pediatr Drugs 14, 417–427 (2012). https://doi.org/10.1007/BF03262422
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF03262422