Abstract
Background
Pharmaceutical companies in Japan often provide a unique document called the Guidance for Proper Usage (GPU) to medical oncologists, in order to promote the proper use of an approved drug.
Aim
The aim of the study was to examine the content of and current trends in the efficacy and safety data provided in GPU documents, in order to understand what pharmaceutical companies regard as important information for an oncologist to ensure proper usage of oncology drugs in Japan. Furthermore, this study analysed the influence of route of administration (oral or injection), drug class (cytotoxic or other), type of malignancy (haematological or solid malignancy) and the period of GPU publication (before and after April 2004) on the content of the GPU document.
Materials and Methods
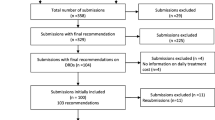
For the 103 oncology drugs approved for use in Japan, 59 GPU documents, covering 50 drugs, were distributed to medical oncologists and were compared by multivariate analysis over two consecutive time periods, from October 1994 to March 2004 and April 2004 to December 2010. For each document, we determined the total number of pages and the proportion of pages that provided drug and toxicity information and the results of registration trials, including postmarketing surveillance.
Results
The median number of pages in the GPUs was 48 (range, 11–98). The proportion of pages providing toxicity information, drug information (e.g. warnings, contraindications, instructions, and directions for indications and usage, dosage and administration) and the results of registration trials were 30%, 26% and 11%, respectively. After April 2004, the publication of GPU documents doubled, and the proportion of pages discussing the results of registration trials significantly increased (p = 0.002). The proportions of pages discussing toxicity information and the results of registration trials in molecular-targeted drugs were significantly greater than those for other types of drugs (p < 0.001 and p = 0.008, respectively), while the proportion of pages discussing drug information was significantly lower (p = 0.001).
Conclusion
The number and length of GPU publications distributed by pharmaceutical companies in Japan has increased since 2004. Significant changes in content have included a reduction in the amount of information about drug administration and an increase in information on the results of pharmaceutical company-sponsored registrational trials. A further study is warranted to evaluate how often oncologists actually access GPU documents and what type of information could be most useful in medical practice in Japan.





Similar content being viewed by others
References
Layton MR, Sritanyarat W, Chadbunchachai S, et al. Sources of information for new drugs among physicians in Thailand. Pharm World Sci 2007; 29(6): 619–27
Japanese Society of Hospital Pharmacists. Main statement point of interview form in 2008 [in Japanese]. J Jpn Soc Hosp Pharm 2009; 45(1): 37–45
Farrell AT, Papadouli I, Hori A, et al. The advisory process for anticancer drug regulation: a global perspective. Ann Oncol 2006; 17(6): 889–96
Pfizer Japan Inc. Guidance for proper usage of sutent. [in Japanese; online]. Available from URL: https://pfizerpro.jp/cs/sv/sutent/product/index.html [Accessed 2012 Mar 3]
Shrank W, Avorn J, Rolon C, et al. Effect of content and format of prescription drug labels on readability, understanding, and medication use: a systematic review. Ann Pharmacother 2007; 41(5): 783–801
Kudoh S, Kato H, Nishiwaki Y, et al. Interstitial lung disease in Japanese patients with lung cancer: a cohort and nested case-control study. Am J Respir Crit Care Med 2008; 177(12): 1348–57
Hotta K, Kiura K, Takigawa N, et al. Comparison of the incidence and pattern of interstitial lung disease during erlotinib and gefitinib treatment in Japanese patients with non-small cell lung cancer: the Okayama Lung Cancer Study Group experience. J Thorac Oncol 2010; 5(2): 179–84
Food and Drug Administration. Risk evaluation mitigation strategy [online]. Available from URL: http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm111350.htm [Accessed 2012 Jan 20]
Fujimoto Pharmaceuticals Corporation. Thalidmide education and risk management system [in Japanese; online]. Available from URL: http://www.fujimotopharm.co.jp/jp/iyakuhin/thalido/index.php [Accessed 2012 Jan 20]
Celgene KK. RevMate (lenalidomide) [in Japanese; online]. Available from URL: http://revmate-japan.jp/index.html [Accessed 2012 Jan 20]
Acknowledgements
This study was supported by a science research grant (H21-005) from the Ministry of Health, Labour and Welfare (for research on infrastructure development for clinical trials). The funding source had no involvement in the study design, conduct, data collection, management, analysis, interpretation, composition, review, or publication of this work. The following provides a list of contributions for this study by the individual authors:
Concept of study: K. Yonemori, Y. Ryushima, A. Hirakawa and Y. Fujiwara. Provision of study material: K. Yonemori, Y. Ryushima, M. Saito and H. Yamamoto. Data collection: K. Yonemori, Y. Ryushima and M. Saito. Statistical analyses: A. Hirakawa. Data interpretation: T. Hirata, H. Yamamoto, M. Ando, A. Hirakawa, C. Shimizu, K. Tamura, M. Yunokawa, M. Kodaira, Y. Fujiwara, H. Yamamoto, Y. Ryushima and M. Saito. Manuscript writing: K. Yonemori, T. Hirata, H. Yamamoto, M. Ando Y. Fujiwara, M. Saitoand Y. Ryushima. Manuscript review: C. Shimizu, K. Tamura, M. Yunokawa, M. Kodaira and H. Yamamoto. Final manuscript approval: K. Yonemori, A. Hirakawa, T. Hirata, H. Yamamoto, M. Ando, C. Shimizu, K. Tamura, M. Yunokawa, M. Kodaira, Y. Fujiwara, H. Yamamoto, Y. Ryushima and M. Saito. Financial and administrative support: Y. Fujiwara. Masashi Ando has received research funding from Sanofi-Aventis, Wyeth, Novartis and GlaxoSmithKline; Chikako Shimizuhas received honoraria from Daiichi Sankyo, Novartis, Chugai, Astra-Zeneca, Sanofi-Aventis and GlaxoSmithKline; Kenji Tamruahas received honoraria from Bristrol Myers Squibb and Chugai and a research grant from Merck Sharp and Dohme. Yasuhiro Fujiwara has received honoraria from Taiho, Sanofi-Aventis, Eli Lilly and Chugai and research funding from Pfizer, GlaxoSmithKline, Chugai, Eisai, Daiichi Sankyo, Taiho, Nihon Kayaku, Amgen, Novartis, Takeda and Astra-Zeneca. The remaining authors have no conflicts of interest to declare that are directly relevant to the content of this study.
This study was presented, in part, at the European Multidisciplinary Cancer Congress (ESMO/ECCO/ESTRO) 2011, in Stockholm, Sweden.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yonemori, K., Hirakawa, A., Ryushima, Y. et al. An Analysis of Guidance for Proper Usage Documents for Oncology Drugs in Japan. Pharm Med 26, 165–170 (2012). https://doi.org/10.1007/BF03262390
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF03262390