Abstract
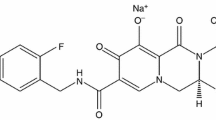
Etravirine (Intelence®), an oral next-generation non-nucleoside reverse transcriptase inhibitor (NNRTI), is approved for the treatment of HIV-1 infection in treatment-experienced patients. In the US, the approved use of etravirine in patients who have evidence of viral replication and harbor HIV-1 strains resistant to other antiretroviral agents has recently been expanded to include pediatric patients aged ≥6 to <18 years. At 24 and 48 weeks in an open-label trial, etravirine 5.2mg/kg twice daily (maximum dosage 200 mg twice daily) plus an optimized background therapy regimen achieved complete viral response (defined as HIV-1 RNA <50 copies/mL) in approximately half of treatment-experienced children and adolescents with HIV-1 infection and evidence of viral replication, and had an acceptable tolerability profile.



Similar content being viewed by others
References
Panel on Antiretroviral Therapy and Medical Management of HIV-Infected Children. Guidelines for the use of antiretroviral agents in pediatric HIV infection. Bethesda (MD): US Department of Health and Human Services, 2011 Aug 11.
Thompson MA, Mugavero MJ, Amico KR, et al. Guidelines for improving entry into and retention in care and antiretroviral adherence for persons with HIV: evidence-based recommendations from an International Association of Physicians in AIDS Care panel. Ann Intern Med 2012 Jun 5; 156 (11): 817–33.
Shet A, Berry L, Mohri H, et al. Tracking the prevalence of transmitted antiretroviral drug-resistant HIV-1: a decade of experience. J Acquir Immune Defic Syndr 2006 Apr 1; 41 (4): 439–46.
Penazzato M, Giaquinto C. Role of non-nucleoside reverse transcriptase inhibitors in treating HIV-infected children. Drugs 2011; 71 (16): 2131–49.
Croxtall JD. Etravirine: a review of its use in the management of treatment-experienced patients with HIV-1 infection. Drugs 2012; 72 (6): 847–69.
Madruga JV, Cahn P, Grinsztejn B, et al. Efficacy and safety of TMC125 (etravirine) in treatment-experienced HIV-1-infected patients in DUET-1:24-week results from a randomised, double-blind, placebo-controlled trial. Lancet 2007 Jul 7; 370 (9581): 29–38.
Lazzarin A, Campbell T, Clotet B, et al. Efficacy and safety of TMC125 (etravirine) in treatment-experienced HIV-1-infected patients in DUET-2: 350 24-week results from a randomised, double-blind, placebo-controlled trial. Lancet 2007 Jul 7; 370 (9581): 39–48.
Katlama C, Haubrich R, Lalezari J, et al. Efficacy and safety of etravirine in treatment-experienced, HIV-1 patients: pooled 48 week analysis of two randomized, controlled trials. AIDS 2009; 23 (17): 2289–300.
Katlama C, Clotet B, Mills A, et al. Efficacy and safety of etravirine at week 96 in treatment-experienced HIV type-1-infected patients in the DUET-1 and DUET-2 trials. Antiviral Ther 2010; 15 (7): 1045–52.
Intelence® (etravirine) tablets for oral use: US prescribing information. Titusville (NJ): Janssen Pharmaceuticals, Inc., 2012 Mar.
Figueiredo A, Moore KL, Mak J, et al. Potent nonnucleoside reverse transcriptase inhibitors target HIV-1 Gag-Pol. PLoS Pathog 2006 Nov; 2 (11): e1 19.
Das K, Clark Jr AD, Lewi PJ, et al. Roles of conformational and positional adaptability in structure-based design of TMC125-R165335 (etravirine) and related non-nucleoside reverse transcriptase inhibitors that are highly potent and effective against wild-type and drug-resistant HIV-1 variants. J Med Chem 2004 May 6; 47 (10): 2550–60.
Vingerhoets J, Tambuyzer L, Azijn H, et al. Resistance profile of etravirine: combined analysis of baseline genotypic and phenotypic data from the randomized, controlled phase III clinical studies. AIDS 2010; 20; 24 (4): 503–14.
Tambuyzer L, Nijs S, Daems B, et al. Effect of mutations at position E138 in HIV-1 reverse transcriptase on phenotypic susceptibility and virologic response to etravirine. J Acquir Immune Defic Syndr 2011 Sep 1; 58 (1): 18–22.
Fulco PP, McNicholl IR. Etravirine and rilpivirine: nonnucleoside reverse transcriptase inhibitors with activity against human immunodeficiency virus type 1 strains resistant to previous nonnucleoside agents. Pharmacotherapy 2009 Mar; 29 (3): 281–94.
Tudor-Williams G, Cahn P, Chokephaibulkit K, et al. Safety and efficacy of etravirine in HIV-1-infected, treatment-experienced children and adolescents: PIANO 48-week results [abstract no. TUAB0204]. AIDS 2012: XIX International AIDS Conference; 2012 Jul 22–27; Washington, DC.
Schöller-Gyüre M, Kakuda TN, Van Solingen-Ristea RM, et al. Bioavailability of the 100 mg etravirine tablet dispersed in water and of the 25 mg pediatric tablet formulation [abstract plus poster no. MOPE0184]. AIDS 2008: XVII International AIDS Conference; 2008 Aug 3–8; Mexico City 18. Kakuda A, Green B, Morrish G, et al. Population pharmacokinetics of etravirine in HIV-1-infected treatment-experienced children and adolescents (6–17 years): week 24 primary analysis of the phase II PIANO trial [abstract plus poster no. TULPE026]. 6th International AIDS Society Conference on HIV Pathogenesis, treatment and prevention; 2011 Jul 17–20; Rome.
Königs C, Feiterna-Sperling C, Esposito S, et al. Pharmacokinetics and short-term safety and tolerability of etravirine in treatment-experienced HIV-infected children and adolescents. AIDS 2012 Feb; 26 (4): 447–55.
Tudor-Williams G, Cahn P, Chokephaibulkit K, Fourie J, et al. Safety and efficacy of HIV-1-infected, treatment-experienced children and adolescents (6–17 years): week 24 primary analysis of the phase II PIANO study [abstract plus poster no. TULPE027]. 6th International AIDS Society Conference on HIV Pathogenesis, treatment and prevention; 2011 Jul 17–20; Rome.
Briz V, Palladino C, Navarro M, et al. Etravirine-based highly active antiretroviral therapy in HIV-1-infected paediatric patients. HIV Med 2011 Aug; 12 (7): 442–6.
AIDS info. Guidelines for the use of antiretroviral agents in pediatric HIV infection [online]. Available from: URL: http://www.aidsinfo.nih.gov/con/lvguidelines/pediatricguidelines.pdf [Accessed 2012 Jun 13]
US National Institutes of Health. ClinicalTrials.gov [online]. Available from URL: http://www.clinicaltrials.gov [Accessed 2012 Jun 7].
Acknowledgments and Disclosures
This article was reviewed by R. ter Heine, Department of Pharmacy and Pharmacology, Slotervaart Hospital, Amsterdam, the Netherlands.
The preparation of this article was not supported by any external funding. During the peer review process, the manufacturer of the agent under review was offered an opportunity to comment on the article. Changes resulting from comments received were made by the authors on the basis of scientific and editorial merit.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lyseng-Williamson, K.A. Etravirine: A Guide to Its Use in Treatment-Experienced Pediatric Patients with HIV-1 Infection in the US. Pediatr Drugs 14, 345–350 (2012). https://doi.org/10.1007/BF03262240
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF03262240