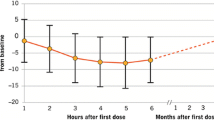
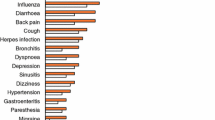
Abstract
Fingolimod, a novel sphingosine 1-phosphate receptor agonist, is indicated for the treatment of patients with relapsing- remitting multiple sclerosis. In clinical trials, oral fingolimod was more effective than placebo and intramuscular interferon β-1a at reducing annualized relapse rates and the burden and activity of disease.



Similar content being viewed by others
References
Scott LJ. Fingolimod: a review of its use in the management of relapsing-remitting multiple sclerosis. CNS Drugs 2011; 25 (8): 673–98
Gilenya: summary of product characteristics. London: European Medicines Agency, 2012 Jul 6
Kappos L, Radue E-W, O’Connor P, et al. A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. N Engl J Med 2010; 362 (5): 387–401
Haas J, Hartung HP, Von Rosenstiel P, et al. Effect of fingolimod on severe multiple sclerosis relapses, healthcare utilization and recovery: results from two phase 3 studies, TRANSFORMS and FREEDOMS [abstract plus poster. no P06.049]. 63rd Annual Meeting of the American Academy of Neurology; 2011 Apr 9–16; Honolulu (HI)
Cohen JA, Barkhof F, Comi G, et al. Oral fingolimod or intramuscular interferon for relapsing multiple sclerosis. N Engl J Med 2010; 362 (5): 402–15
Calabresi PA, Radue E-W, Goodin D, et al. Efficacy and safety of fingolimod in patients with relapsing remitting multiple sclerosis (RRMS): results from an additional 24-month double-blind, placebo-controlled study (FREEDOMS II study) [poster no. E-002]. 64rd Annual Meeting of the American Academy of Neurology; 2012 Apr 21–28; New Orleans (LA)
Cohen J, Barkhof F, Comi G, et al. Oral fingolimod (FTY720) treatment improves the performance of daily activities compared with intramuscular interferon β-1a: Patient-Reported Indices for Multiple Sclerosis (PRIMUS)-Activities results from a phase III study (TRANSFORMS) [abstract no. P06.137]. 62nd Annual Meeting of the American Academy of Neurology; 2010 Apr 10–17; Toronto (ON)
Khatri B, Barkhof F, Comi G, et al. Comparison of fingolimod with interferon beta-1a in relapsing-remitting multiple sclerosis: a randomised extension of the TRANSFORMS study. Lancet Neurol 2011; 10 (6): 520–9
Izquierdo G, O’Connor P, Montalban X, et al. Long-term fingolimod (FTY720) in relapsing MS: 5 years results from an extension of a phase II, multicenter study show a sustained low level of disease activity [abstract plus poster no. PD6.004]. 63rd Annual Meeting of the American Academy of Neurology; 2011 Apr 9–16; Honolulu (HI)
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Scott, L.J., Lyseng-Williamson, K.A. Fingolimod: a guide to its use in multiple sclerosis in the EU. Drugs Ther Perspect 28, 6–11 (2012). https://doi.org/10.1007/BF03262126
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF03262126