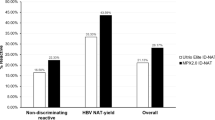
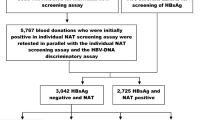
Abstract
Background: Nucleic acid amplification technology (NAT) is presently being evaluated in US clinical trials to determine the safety and efficacy of mini-pool testing for human immunodeficiency virus (HIV) and hepatitis C virus (HCV) RNA in the blood-donor population. Although the risk for transfusion-transmitted HIV and HCV infection is extremely low, there is still a small chance that blood donated by infected individuals before seroconversion can escape detection by current antibody-based assays.
Methods: This report describes the amplification technologies being used and reviews several issues surrounding NAT-based blood screening. The performance features of NAT and current enzyme immunoassay technologies are compared, and the benefits of NAT in reducing transfusion-transmitted infections are discussed.
Conclusions: The current US clinical trials of mini-pool NAT testing for HIV and HCV RNA have successfully identified preseroconversion infectious blood units. Although the current NAT-based screening systems are semiautomated, mini-pool testing represents an unprecedented innovation among government and nongovernment agencies in the highly regulated blood transfusion industry. Despite cost-effectiveness issues, based on the public perception of infectious diseases acquired through blood transfusion, NAT-based screening of the blood supply is expected to become a standard in transfusion medicine.
Similar content being viewed by others
References
Centers for Disease Control: Update on acquired immunodeficiency syndrome (AIDS) among patients with hemophilia A. Morb Mortal Wkly Rep 1982; 31: 644-651
Beeson PB: Jaundice occurring one to four months after transfusion of blood or plasma. Report of seven cases. JAMA 1943;121: 1332–1334
Hewlett IK, Epstein JS: Food and Drug Administration conference on the feasibility of genetic technology to close the HIV window in donor screening (conference report). Transfusion 1997;37: 346–351
Gallarda JL, Henrard DR, Liu D, et al.: Early detection of antibody to human immunodeficiency virus type 1 by an antigen conjugate immunoassay correlates with the presence of IgM antibody. sJ Clin Microbiol 1992;30: 770–772
Schreiber GB, Busch MP, Kleinman SH, Korelitz JJ: The risk of transfusion-transmitted viral infections. The Retrovirus Epidemiology Donors Study. N Engl J Med 1996;334: 1685–1690
Busch MP, Lee LL, Satten GA, et al.: Time course of detection of viral and serologic markers preceding human immunodeficiency virus type 1 seroconversion: Implications for screening blood and tissue donors. Transfusion 1995;35: 91–97
Busch MP, Satten GA, Herman SA, et al.: Time course and kinetics of HIV viremia during primary infection. Transfusion 1996;37: S41A (suppl, abstr)
Busch MP, Stramer S, Kleinman S: In Garrety G: Evolving applications of nucleic acid amplification assays for prevention of virus transmission by blood components and derivatives. Applications of molecular biology to blood transfusion medicine. American Association of Blood Banks, Bethesda, 1997
Lelie PN, Cuypers HT, Reesink HW, et al.: Patterns of serological markers in transfusion-transmitted hepatitis C virus infection using second generation HCV assays. J Med Virol 1992;37: 203–209
Busch MP, Korelitz LL, Kleinman SH, et al.: Declining value of alanine aminotransferase in screening of blood donors to prevent post-transfusion hepatitis B and C virus infection. Transfusion 1995; 35: 903–910
Alter HJ: To C or not to C: These are the questions. Blood 1995;85: 1681–1695
Kleinman SH, Busch MP, Korelitz JJ, Schreiber GB: The incidence/window period model and its use to assess the risk of transfusion-transmitted human immunodeficiency virus and hepatitis C virus infection. Transfus Med Rev 1997;11: 155–172
Barrera JM, Francis B, Ercilla G, et al.: Improved detection of anti-HCV in post-transfusion hepatitis by a third generation ELISA. Vox Sang 1995;68: 15–18
Murthy KK, Henrard DR, Eichberg JW, et al: Redefining the HIV-infectious window period in the chimpanzee model: Evidence to suggest that viral nucleic acid testing can prevent blood-borne transmission. Transfusion 1999;39: 688–693
Leviton LB, Sox HC Jr, Stoto MA: HIV and the blood supply. An analysis of crisis decision making. Committee to study HIV transmission through blood and blood products. Division of Health Promotion and Disease Prevention, Institute of Medicine, National Academy Press, Washington, 1995
Shays C: Safety of the blood supply must become a priority. Disaster of the early 1980s, when more than 300,000 patients were infected because of bad blood, cannot be repeated. Capital Hill Roll Call, March 1997
Committee for Proprietary Medicinal Products: The application of genome amplification technology (GAT) to plasma fractionation pools. CPMP/BWP/ 390/97 (rev 6)
Rogers PM, Saldanha J, Allain JP: Report of EPFA/ NIBSC Workshop, nucleic acid amplification tests (NAT) for the detection of blood-borne viruses. Amsterdam, October 1996. Vox Sang 1997;72: 199–206
Billyard B, Acedo M, Friedenberg M, et al.: A high-throughput, semi-automated HIV-1/HCV system for screening pooled plasma. American Association of Blood Banks 51st Annual Meeting. October 31–November 3, 1998, Philadelphia, PA (poster presentation
Kacian DL, Fultz TZ: Nucleic acid sequence amplification methods. US patent 5,399,491, March 21, 1995
McDonough SH, Giachetti C, Yang Y, Kolk DP, Billyard E, Mimms L: High throughput assay for the simultaneous or separate detection of human immunodeficiency virus (HIV) and hepatitis type c virus (HCV). Infusion Ther Transfus Med 1998;25: 164–169
Arnold LJ, Hammond PW, Wiese WA, Nelson NC: Assay formats involving acridinium-ester-labeled DNA probes. Clin Chem 1989;35: 1588–1594
Saade C, Wust T: Specificity of a new test for detection of antibodies to HIV-1/2 in blood donors. Infusion Ther Transfus Med 1996;23: 133–137
Buffington J, Shapiro CN, Holman RC, et al.: Multiple unconfirmed-reactive screening tests for antibodies among blood donors. Transfusion 1994;34: 371–375
Silvester C, Healey DS, Cunningham P, Dax EM: Multisite evaluation of four anti-HIV-1/HIV-2 enzyme immunoassays. sAustralian HIV Test Evaluation Group. J Acquir Immune Defic Syndr Hum Retrovirol 1995;8: 411–419
Henrard D, Phillips J, Windsor I, et al.: Detection of human immunodeficiency virus type 1 p24 antigen and plasma RNA: Relevance to indeterminate serologic tests. Transfusion 1994;34: 376–380
Busch MP, Kleinman SH, Williams AE, et al.: Frequency of human immunodeficiency virus (HIV) infection among contemporary anti-HIV-1 and anti-HIV-1/2 supplemental test-indeterminate blood donors.Transfusion 1996;36: 37–44
McCullough J, Bianco C, Busch MP, et al.: Interagency Genome Amplification Task Force: Preliminary report. Transfusion 1998;38: 903–904
Dorfman R: The detection of defective members of large populations. Ann Math Stat 1943; 14: 436–440
Litvak E, Xin MT, Pagano M: Screening for the presence of a disease by pooling sera samples. J Am Stat Assoc 1994;89: 424–434
Archbold E, Mitchel S, Hanson D, Hull B, Galo LM, Monzon O: Serum-pooling strategies for HIV screening: Experiences in Trinidad, Ecuador, and the Phillipines. VII Int Conf AIDS 1991 Florence, Italy; 2: 331 (abstr)
Behets F, Bertozzi S, Kasali M, et al.: Successful use of pooled sera to determine HIV-1 seroprevalence in Zaire with development of cost-efficiency models. AIDS 1990;4: 737–741
Calhoon-Young B, Chandler A, Livermore T, Gaudino J, Benjamin R: Sensitivity and specificity of pooled versus individual sera in a human immunodeficiency virus (HIV) antibody prevalence study. J Clin Microbiol 1989;27: 1893–1895
Kline RL, Brothers TA, Brookmeyer R, Zeger S, Quinn TC: Evaluation of human immunodeficiency virus (HIV) seroprevalence in population surveys using pooled sera. J Clin Microbiol 1989;27: 1449–1455
Sanchez M, Leoro G, Archbold E: Workload and cost-effectiveness analysis of a pooling method for HIV screening. sVII Int Conf AIDS 1991;2: 330 (abstr)
Cagliotti S. McAuley J, Spizman R, Busch MP: Performance of the Chiron/Gen-Probe HIV-1/HCV TMA assay in a high-volume NAT laboratory. American Association of Blood Banks 52nd Annual Meeting November 6–11, 1999, San Francisco, CA (abstr)
Longo MC, Berninger MS, Hartley JL: The use of uracil DNA glycosylase to control carry-over contamination in polymerase chain reactions. Gene 1990;93: 125–128
Rayfield MA, Sullivan P, Bandea CI, et al.: HIV-1 group O virus identified for the first time in the United States. Emerg Infect Dis 1996;2: 209–212
Sullivan MT, Guido EA, Metier RP, Schable CA, Williams AE, Stramer SL: Identification and characterization of an HIV-2 antibody-positive blood donor in the United States. Transfusion 1998;38: 189–193
Busch MP, Chamberland M, Epstein J, Kleinman S, Khabbaz R, Nemo G: Oversight and monitoring of blood safety in the United States. Vox Sang 1999; 77: 67–76
Food and Drug Administration: Center for Biologics Evaluation and Research: Memo to All Registered Blood and Plasma Establishments. Rockville, MD, August 8, 1995
Aubuchon JP, Birkmeyer JD, Busch MP: Cost-effectiveness of expanded human immunodeficiency virus-testing protocols for donated blood. Transfusion 1997;37: 45–51
Busch MP, Stramer SL: The efficiency of HIV p24 antigen screening of US blood donors: Projections versus reality. Infusion Ther Transfus Med 1998;25: 5–8
Aubuchon JP, Birkmeyer JD, Busch MP: Safety of the blood supply in the United States: Opportunities and controversies. Ann Intern Med 1997;127: 904–990
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gallarda, J.L., Dragon, E. Blood Screening by Nucleic Acid Amplification Technology: Current Issues, Future Challenges. Molecular Diagnosis 5, 11–22 (2000). https://doi.org/10.1007/BF03262018
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF03262018