Abstract
Vemurafenib is a first-in-class, small molecule BRAFV600E inhibitor. It is indicated in the US for the treatment of patients with unresectable or metastatic melanoma with the BRAFV600E mutation, and in the EU as monotherapy in adults with BRAFV600 mutation-positive unresectable or metastatic melanoma.
Oral vemurafenib improved overall survival (OS) [co-primary endpoint] in patients with unresectable, previously untreated, BRAFV600E mutation-positive, stage IIIC or IV melanoma, according to the results of a randomized, open-label, multicenter, phase III trial (BRIM-3). With vemurafenib versus dacarbazine, the risk of death was significantly reduced by 63% in the interim OS analysis, and by 56%, 38%, and 30% in subsequent updated OS analyses. The median OS duration was 13.6 months in vemurafenib recipients and 9.7 months in dacarbazine recipients in the most recent OS analysis.
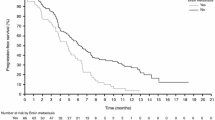
In the phase III trial, progression-free survival (PFS) [co-primary endpoint] was also significantly improved in vemurafenib versus dacarbazine recipients (median PFS of 5.3 vs 1.6 months), with a significant reduction in the risk of death or disease progression of 74% in the final PFS analysis.
Vemurafenib was also associated with a high overall response rate in patients with previously treated, BRAFV600 mutation-positive, stage IV melanoma, according to the results of a noncomparative, multicenter, phase II trial. Patients had received at least one prior systemic treatment for advanced disease (excluding BRAF inhibitors other than sorafenib or MEK inhibitors). The overall response rate (primary endpoint) was 53% (complete response rate of 6% and partial response rate of 47%), with a median duration of response of 6.7 months, and a median OS duration of 15.9 months.
Oral vemurafenib was generally well tolerated in patients with metastatic melanoma, with cutaneous adverse events among the most commonly occurring adverse events. Cutaneous squamous cell carcinoma and/or keratoacanthoma were reported in 18% of vemurafenib recipients in the BRIM-3 trial.



Similar content being viewed by others
References
Lemech C, Arkenau H-T. Novel treatments for metastatic cutaneous melanoma and the management of emergent toxicities. Clin Med Insights Oncol 2012; 6: 53–66
National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology (NCCN guidelines®): melanoma (version 3.2012) [online]. Available from URL: http://www.nccn.org/professionals/physician_gls/pdf/melanoma.pdf [Accessed 2012 May 31]
Finn L, Markovic SN, Joseph RW. Therapy for metastatic melanoma: the past, present, and future. BMC Med 2012; 10: 23
Serrone L, Zeuli M, Sega FM, et al. Dacarbazine-based chemotherapy for metastatic melanoma: thirty-year experience overview. J Exp Clin Can Res 2000 Mar; 19(1): 21–34
Manousaridis I, Mavridou S, Goerdt S, et al. Cutaneous side effects of inhibitors of the RAS/RAF/MEK/ERK signalling pathway and their management. J Eur Acad Dermatol Venereol. Epub 2012 Apr 28
Natarajan N, Telang S, Miller D, et al. Novel immunotherapeutic agents and small molecule antagonists of signalling kinases for the treatment of metastatic melanoma. Drugs 2011; 71(10): 1233–50
Ascierto PA, Kirkwood JM, Grob J-J, et al. The role of BRAF V600 mutation in melanoma. J Transl Med 2012 May 3; 10(1): 85
Kudchadkar R, Paraiso KHT, Smalley KSM. Targeting mutant BRAF in melanoma: current status and future development of combination therapy strategies. Cancer J. 2012 Mar/Apr; 18(2): 124–31
Flaherty KT, Puzanov I, Kim KB, et al. Inhibition of mutated, activated BRAF in metastatic melanoma. N Engl J Med 2010 Aug 26; 363(9): 809–19
Yang H, Higgins B, Kolinsky K, et al. RG7204 (PLX4032), a selective BRAFV600E inhibitor, displays potent antitumor activity in preclinical melanoma models. Cancer Res 2010 Jul 1; 70(13): 5518–27
European Medicines Agency. Zelboraf (vemurafenib) 240 mg film-coated tablets: EU summary of product characteristics [online]. Available from URL: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002409/WC500124317.pdf [Accessed 2012 May 29]
Tsai J, Lee JT, Wang W, et al. Discovery of a selective inhibitor of oncogenic B-Raf kinase with potent antimelanoma activity. Proc Natl Acad Sci U S A 2008 Feb 26; 105(8): 3041–6
Vergani E, Vallacchi V, Frigerio S, et al. Identification of MET and SRC activation in melanoma cell lines showing primary resistance to PLX4032. Neoplasia 2011 Dec; 13(12): 1132–42
Genentech Inc. Zelboraf® (vemurafenib) tablet, oral: US prescribing information [online]. Available from URL: http://www.gene.com/gene/products/information/zelboraf/pdf/pi.pdf [Accessed 2012 May 29]
Halait H, DeMartin K, Shah S, et al. Analytical performance of a real-time PCR-based assay for V600 mutations in the BRAF gene, used as the companion diagnostic test for the novel BRAF inhibior vemurafenib in metastatic melanoma. Diag Mol Pathol 2012 Mar; 21(1): 1–8
Sala E, Mologni L, Truffa S, et al. BRAF silencing by short hairpin RNA or chemical blockade by PLX4032 leads to different responses in melanoma and thyroid carcinoma cells. Mol Cancer Res 2008 May; 6(5): 751–9
Søndergaard JN, Nazarian R, Wang Q, et al. Differential sensitivity of melanoma cell lines with BRAFV600E mutation to the specific Raf inhibitor PLX4032. J Transl Med 2010; 8: 39
Meier FE, Beck D, Niessner H, et al. Induction of apoptosis by the BRAFV600E kinase inhibitor PLX4032 in BRAFV600E melanoma cells through regulation of endoplasmic reticulum stress-related genes [abstract no. 8518]. 47th Annual Meeting of the American Society of Clinical Oncology; 2011 Jun 3–7; Chicago (IL)
McArthur GA, Puzanov I, Amaravadi R, et al. Marked, homogeneous, and early [18F]fluorodeoxyglucose-positron emission tomography responses to vemurafenib in BRAF-mutant advanced melanoma. J Clin Oncol 2012 May 10; 30(14): 1628–34
Sosman JA, Kim KB, Schuchter L, et al. Survival in BRAF V600-mutant advanced melanoma treated with vemurafenib. N Engl J Med 2012 Feb 23; 366(8): 707–14
Chapman PB, Hauschild A, Robert C, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med 2011 Jun 30; 364(26): 2507–16
Mittapalli RK, Vaidhyanathan S, Sane R, et al. Impact of P-glycoprotein (ABCB1) and breast cancer resistance protein (ABCG2) on the brain distribution of a novel B-RAF inhibitor: vemurafenib (PLX4032). J Pharmacol Exp Ther 2012 Jul; 342(1): 33–40
Dummer R, Rinderknecht J, Goldinger SM, et al. An open-label pilot study of vemurafenib in previously treated metastatic melanoma patients with brain metastases [abstract no. 8548]. 47th Annual Meeting of the American Society of Clinical Oncology; 2012 Jun 3–7; Chicago (IL)
Hoffmann-La Roche. A pharmacokinetic study of RO5185426 in combination with a drug cocktail in patients with metastatic melanoma [ClinicalTrials.gov identifier NCT01001299]. US National Institutes of Health, Clinical-Trials.gov [online]. Available from URL: http://www.clinicaltrials.gov [Accessed 2012 Jun 1]
McArthur G, Hauschild A, Robert C, et al. Vemurafenib improves overall survival compared to dacarbazine in advanced BRAFV600E-mutated melanoma: updated survival results from a phase III randomized, open-label, multicentre trial [abstract]. European Multidisciplinary Cancer Congress 2011; 2011 Sep 23–27; Stockholm
Chapman PB, Hauschild A, Robert C, et al. Updated overall survival (OS) results for BRIM-3, a phase III randomized, open-label, multicenter trial comparing BRAF inhibitor vemurafenib (vem) with dacarbazine (DTIC) in previously untreated patients with BRAFV600E-mutated melanoma [abstract no. 8502 plus oral presentation]. 48th Annual Meeting of the American Society of Clinical Oncology; 2012 Jun 1–5; Chicago (IL)
Schucter LM, Flaherty LE, Hamid O, et al. A single-arm, open-label, U.S. expanded access study of vemurafenib in patients with metastatic melanoma [abstract no. 8567]. 48th Annual Meeting of the American Society of Clinical Oncology; 2012 Jun 1–5; Chicago (IL)
Wagle N, Emery C, Berger MF, et al. Dissecting therapeutic resistance to RAF inhibition in melanoma by tumor genomic profiling. J Clin Oncol 2011 Aug 1; 29(22): 3085–96
Paraiso KHT, Xiang Y, Rebecca VW, et al. PTEN loss confers BRAF inhibitor resistance to melanoma cells through the suppression of BIM expression. Cancer Res 2011 Apr 1; 71(7): 2750–60
Nazarian R, Shi H, Wang Q, et al. Melanomas acquire resistance to B-RAF(V600E) inhibition by RTK or N-RAS upregulation. Nature 2010 Dec 16; 468(7326): 973–7
Villanueva J, Vultur A, Lee JT, et al. Acquired resistance to BRAF inhibitors mediated by a RAF kinase switch in melanoma can be overcome by cotargeting MEK and IGF-1R/PI3K. Cancer Cell 2010 Dec 14; 18(6): 683–95
Atefi M, von Euw E, Attar N, et al. Reversing melanoma cross-resistance to BRAF and MEK inhibitors by co-targeting the AKT/mTOR pathway. PLoS ONE2011Dec; 6 (12): e28973
Poulikakos PI, Persaud Y, Janakiraman M, et al. RAF inhibitor resistance is mediated by dimerization of aberrantly spliced BRAF(V600E). Nature 2011 Dec 15; 480(7377): 387–90
Johannessen CM, Boehm JS, Kim SY, et al. COT drives resistance to RAF inhibition through MAP kinase pathway reactivation. Nature 2010 Dec 16; 468(7326): 968–72
Yadav V, Zhang X, Liu J, et al. Reactivation of mitogen-activated protein kinase (MAPK) pathway by FGF receptor 3 (FGFR3)/Ras mediates resistance to vemurafenib in human B-RAF V600E mutant melanoma. J Biol Chem. Epub 2012 Jun 22
Niehr F, von Euw E, Attar N, et al. Combination therapy with vemurafenib (PLX4032/RG7204) and metformin in melanoma cell lines with distinct driver mutations. J Transl Med 2011; 9: 76
Shi H, Kong X, Ribas A, et al. Combinatorial treatments that overcome PDGFRb-driven resistance of melanoma cells to V600EB-RAF inhibition. Cancer Res 2011 Aug 1; 71(15): 5067–74
Larkin JMG, Queirolo P, Arance AM, et al. An open-label, multicenter safety study of vemurafenib (PLX4032, RO5185426) in patients with metastatic melanoma [abstract no. 8517]. 48th Annual Meeting of the American Society of Clinical Oncology; 2012 Jun 1–5; Chicago (IL)
Su F, Viros A, Milagre C, et al. RAS mutations in cutaneous squamous-cell carcinomas in patients treated with BRAF inhibitors. N Engl J Med 2012 Jan 19; 366(3): 207–15
Oberholzer PA, Kee D, Dziunycz P, et al. RAS mutations are associated with the development of cutaneous squamous cell tumors in patients treated with RAF inhibitors. J Clin Oncol 2012 Jan 20; 30(3): 316–21
Ravnan MC, Matalka MS. Vemurafenib in patients with BRAF V600E mutation-positive advanced melanoma. Clin Ther 2012 Jul; 34(7): 1474–86
Infante JR, Falchook GS, Lawrence DP, et al. Phase I/II study to assess safety, pharmacokinetics, and efficacy of the oral MEK 1/2 inhibitor GSK1120212 (GSK212) dosed in combination with the oral BRAF inhibitor GSK2118436 (GSK436) [abstract no. CRA8503]. 47th Annual Meeting of the American Society of Clinical Oncology; 2011 Jun 3–7; Chicago (IL)
Alloo A, Garibyan L, LeBoeuf N, et al. Photodynamic therapy for multiple eruptive keratoacanthomas associated with vemurafenib treatment for metastatic melanoma. Arch Dermatol 2012 Mar; 148(3): 363–6
Anforth R, Blumetti TCMP, Affandi AM, et al. Systemic retinoid therapy for chemoprevention of nonmelanoma skin cancer in a patient treated with vemurafenib. J Clin Oncol. Epub 2012 Jun 4
Ribas A, Hodi S, Kurland JF, et al. CA184-161: a phase I/II trial of vemurafenib and ipilimumab in patients with BRAF V600 mutation-positive metastatic melanoma [abstract TPS8603]. 48th Annual Meeting of the American Society of Clinical Oncology; 2012 Jun 1–5; Chicago (IL)
Kantor A, Daud A, Munster PN, et al. A phase I/II trial of BKM120 combined with vemurafenib (PLX4032) in BRAFV600E/k mutant advanced melanoma [abstract no. TPS8602]. 48th Annual Meeting of the American Society of Clinical Oncology; 2012 Jun 1–5; Chicago (IL)
Hoffmann-La Roche. A study of vemurafenib in metastatic melanoma patients with brain metastases [ClinicalTrials.gov identifier NCT01378975]. US National Institutes of Health, ClinicalTrials.gov [online]. Available from URL: http://www.clinicaltrials.gov [Accessed 2012 Jul 23]
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Keating, G.M. Vemurafenib. BioDrugs 26, 325–334 (2012). https://doi.org/10.1007/BF03261890
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF03261890