Abstract
Introduction: Hereditary persistence of fetal hemoglobin (HPFH) is a benign condition caused by the failure of normal switching from the fetal to the adult β-globin gene, resulting in continuous production of fetal hemoglobin beyond the perinatal period. To date, eight deletions of variable size and position have been reported for HPFH. Southern hybridization and PCR are the most common methods used to detect each deletion.
Aim: Our aim was to develop a multiplex-PCR assay to detect these deletions in a single tube in order to facilitate rapid and accurate molecular diagnosis.
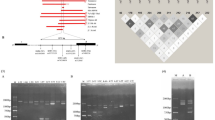
Methods and results: This report is the first application of multiplex-gap-PCR to detect all HPFH deletions simultaneously to expedite diagnosis. The deletion breakpoints were precisely identified for each deletion and primers were designed in the unique regions across the breakpoints of HPFH-1 (Black), HPFH-2 (Ghanaian), HPFH-3 (Asian Indian), HPFH-4 (Italian), HPFH-5 (Italian), HPFH-6 (Vietnamese), HPFH-7 (Kenyan), and SEA-HPFH (Southeast Asian). As many as 16 primers were used in a single amplification reaction by adjusting the relative primer concentrations. The multiplex-PCR approach was standardized on known positive control samples. We identified unique deletion-specific products for each deletion. The results were confirmed by sequence analysis.
Conclusions: We conclude that our multiplex-gap PCR strategy provides the most rapid and accurate diagnosis for the deletions in the β-globin gene cluster causing HPFH.



Similar content being viewed by others
References
Stamatoyannopoulous G, Grosveld F. Hemoglobin switching. In: Stamatoyanno-poulous G, Majerus P, Perlmutter R, et al., editors. The molecular basis of blood diseases. Philadelphia (PA): WB Saunders, 2001: 135–82
Forget BG. Molecular basis of hereditary persistence of fetal hemoglobin. Ann N Y Acad Sci 1998; 850: 38–44
Anagnou NP, Perez-Stable C, Gelinas R, et al. Sequences located 3′ to the breakpoint of the hereditary persistence of fetal hemoglobin-3 deletion exhibit enhancer activity and can modify the developmental expression of the human fetal A gamma-globin gene in transgenic mice. J Biol Chem 1995; 270: 10256–63
Calzolari R, McMorrow T, Yannoutsos N, et al. Deletion of a region that is a candidate for the difference between the deletion forms of hereditary persistence of fetal hemoglobin and delta-beta thalassemia affects beta-but not gamma-globin gene expression. EMBO J 1999; 18: 949–58
Feingold EA, Forget BG. The breakpoint of large deletion causing hereditary persistence of fetal hemoglobin occurs within a erythroid DNA domain remote from the β-globin gene cluster. Blood 1989; 74: 2178–86
Bernards R, Flavell RA. Physical mapping of the globin gene deletion in hereditary persistence of foetal hemoglobin (HPFH). Nucleic Acids Res 1980; 8: 1521–34
Gribnau J, Diderich K, Pruzina S, et al. Intergenic transcription and developmental remodeling of chromatin subdomains in the human beta-globin locus. Mol Cell 2005; 5: 377–86
Henthron PS, Smithies O, Mager DL. Molecular analysis of deletions in the human β-globin gene cluster: deletion junction and location of breakpoints. Genomics 1990; 6: 226–37
Kutlar A, Gardiner MB, Headlee MG, et al. Heterogeneity in the molecular basis of three types of hereditary persistence of fetal hemoglobin and the relative synthesis of the Gy and Ay types of γ-chains. Biochem Genet 1984; 22: 21–35
Henthron PS, Mager DL, Smithies O, et al. A gene deletion ending within a complex array of repeated sequences 3′ to the human β-gene cluster. Proc Natl Acad Sci U S A 1986; 83: 5194–8
Saglio G, Camaschella C, Serra A, et al. Italian type of deletional hereditary persistence of fetal hemoglobin. Blood 1986; 68: 646–51
Camaschella C, Serra A, Gottardi E, et al. A new hereditary persistence of fetal hemoglobin deletion has the breakpoint with in the 3′β-globin gene enhancer. Blood 1990; 75: 1000–5
Kosteas T, Palena A, Anagnou NP. Molecular cloning of breakpoints of hereditary persistence of fetal hemoglobin type-6 (HPFH-6) deletion and sequence analysis of the novel juxtaposed region from the 3′ end of the β-globin gene cluster. Hum Genet 1997; 100: 441–5
Xu X-M, Li Z-Q, Liu Z-Y, et al. Molecular characterization and PCR detection of a deletional HPFH: application to rapid prenatal diagnosis for compound heterozygotes of this effect with β-thalassemia in a Chinese family. Am J Hematol 2000; 65: 183–8
Waye JS, Cai S-P, Eng B, et al. Clinical course and molecular characterization of a compound heterozygote for sickle cell hemoglobin and hemoglobin Kenya. Am J Hematol 1992; 41: 289–91
Huisman THJ, Carver MFH, Baysal E. A syllabus of thalassemia mutations 1-309. Augusta (GA): The Sickle Cell Anemia Foundation, 1997
Craig JE, Barnetson RA, Prior J, et al. Rapid detection of deletions causing 6β thalassemia and hereditary persistence of fetal hemoglobin by enzymatic amplification. Blood 1994; 83: 1673–82
Collins FS, Cole JL, Lockwood WK, et al. The deletion in both common types of hereditary persistence of fetal hemoglobin is approximately 105 kilobases. Blood 1987; 70: 1797–03
Cunningham JM, Jane SM. Hemoglobin switching and fetal hemoglobin reactivation. Semin Hematol 1996; 33: 9–23
Bollenkens JA, Forget BG. Δβ-Thalassemia and hereditary persistence of fetal hemoglobin. Hematol Oncol Clin North Am 1991; 5: 399–22
Katsantoni EZ, Langeveld A, Wai AWK, et al. Persistent γ-globin expression in adult transgenic mice is mediated by HPFH-2, HPFH-3, and HPFH-6 breakpoint sequences. Blood 2003; 102: 3412–9
Heeney MW, Delgrosso K, Robinson R, et al. Interpretation of fetal hemoglobin only on newborn screening for hemoglobinopathy. J Pediatr Hematol Oncol 2002; 24: 499–502
Weatherall DJ, Clegg JB. The thalassemia syndrome. 4th ed. Oxford: Blackwell Science, 2001
Garner C, Dew TK, Sherwood R, et al. Heterocellular hereditary persistence of fetal hemoglobin affects the haematological parameters of β-thalassemia trait. Br J Haematol 2003; 123: 353–8
Ghani R, Manji MA, Ahmed N. Hemoglobinopathies among five major ethnic groups in Karachi, Pakistan. Southeast Asian J Trop Med Public Health 2002; 33: 855–61
Costa VA, Acedo MJ, Polimeno NC, et al. Estimation of the frequency of hereditary persistence of fetal hemoglobin in brazil. Cad Saude Publica 2002; 18: 1469–71
Acknowledgements
We thank Professor Anagnou and Dr Theodoros Kosteas, Department of Basic Sciences, Institute of Molecular Biology and Biotechnology, Heraklion, Greece, for providing positive controls for HPFH-4, -5, and -6, and Dr Xiang-Min Xu, Department of Cellular Biology and Medical Genetics, First Military Medical University, Guangzhou, People’s Republic of China, for providing a DNA sample for SEA-HPFH deletion.
No federal sources of funding were used to assist in the preparation of this study. The authors have no conflicts of interest that are directly relevant to the content of this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bhardwaj, U., McCabe, E.R.B. Multiplex-PCR Assay for the Deletions Causing Hereditary Persistence of Fetal Hemoglobin. CNS Drugs 9, 151–156 (2005). https://doi.org/10.1007/BF03260083
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF03260083