Summary
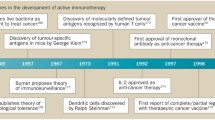
Immunological treatment of malignant tumours is based on the inherent immunogenicity of tumours. Apart from some exceptions, however, tumours of humans are only weakly immunogenic. They induce an immune response that, after skin tests with autologous tumour cells, becomes primarily evident as sensitisation of T lymphocytes to different types of tumour-associated antigens.
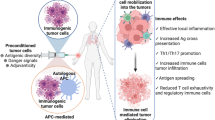
Active specific immunisation pursues the aim of amplifying this pre-existing sensitisation into an effective antitumour immune response. Besides adjuvants there are several ways of increasing the immunogenicity of tumour cells and soluble tumour-associated antigens. However, newly developed strategies to prepare tumour vaccines, such as transfection of tumour cells with the genes of immunological effector molecules or insertion of tumour-associated antigen genes into viral or mycobacterial vectors, may be more effective.
Current clinical trials of active specific immunisation, still highly experimental, are focused especially on patients with malignant melanoma or colorectal carcinoma. Their results may nourish the hopes of clinicians, but cannot yet refute the objections of sceptics.
Similar content being viewed by others
References
Burdick JF, Wells Jr SA, Herberman RB. Immunologic evaluation of patients with cancer by delayed hypersensitivity reactions. Surg Gynecol Obstet 1975; 141: 779–94
Bohle W, Schlag P, Liebrich W, et al. Postoperative active specific immunization in colorectal cancer patients with virus-modified autologous tumour-cell vaccine. Cancer 1990; 66: 1517–23
Bull DM, Leibach JR, Williams MA, et al. Immunity to colorectal cancer assessed by antigen-induced inhibition of mixed mononuclear cell migration. Science 1973; 181: 957–9
Elias EG, Elias LL, Didolkar MS. Cellular immunity in patients with colorectal adenocarcinoma measured by autologous leukocyte migration inhibition. Cancer 1977; 40: 687–92
Old LJ. Cancer immunology: the search for specificity. Cancer Res 1981; 41: 361–75
Rettig WJ. Immunogenetics of cell surface antigens of human cancer. Curr Opin Immunol 1992; 4: 630–40
Chism SE, Burton RC, Warner NL. Immunogenicity of oncofetal antigens: a review. Clin Immunol Immunopathol 1978; 11: 346–77
Zöller M, Matzku S, Schulz U, et al. Sensitization of leukocytes of cancer patients against fetal antigens: leukocyte migration studies. J Natl Cancer Inst 1979; 63: 285–93
Springer GF. T and Tn, general carcinoma autoantigens. Science 1984; 224: 1198–206
Hakomori S. Aberrant glycosylation in cancer cell membranes as focused on glycolipids: overview and perspective. Cancer Res 1985; 45: 2405–14
Kjeldsen T, Clausen H, Hirohashi S, et al. Preparation and characterization of monoclonal antibodies directed to the tumour-associated O-linked sialosyl-2→6α-N-acetylgalactosaminyl (sialosyl-Tn) epitope. Cancer Res 1988; 48: 2214–20
Tai T, Cahan LD, Tsuchida T, et al. Immunogenicity of melanoma-associated gangliosides in cancer patients. Int J Cancer 1985; 35: 607–12
Livingston PO, Natoli Jr EJ, Jones Calves M, et al. Vaccines containing purified GM2 antibodies in melanoma patients. Proc Natl Acad Sci USA 1987; 84: 2911–5
Kodera Y, Bean MA. Antibody-dependent cell-mediated cytotoxicity for human monolayer target cells bearing blood group and transplantation antigens and for melanoma cells. Int J Cancer 1975; 16: 579–92
Shaw ARE, Dasgupta MK, Kovithavongs T, et al. Humoral and cellular immunity to paternal antigens in trophoblastic neoplasia. Int J Cancer 1979; 24: 586–93
Pearson GR, Johansson B, Klein G. Antibody-dependent cellular cytotoxicity against Epstein-Barr virus-associated antigens in African patients with nasopharyngeal carcinomas. Int J Cancer 1978; 22: 120–5
Chan SH, Levine PH, De The GB, et al. A comparison of the prognostic value of antibody-dependent lymphocyte cytotoxicity and other EBV antibody assays in Chinese patients with nasopharyngeal carcinoma. Int J Cancer 1979; 23: 181–5
Jondal M, Svedmyr E, Klein G, et al. Killer T cells in a Burkitt’s lymphoma biopsy. Nature 1975; 155: 405–7
Galili U, Klein E, Klein G, et al. Activated T lymphocytes in infiltrates and draining lymph nodes of nasopharyngeal carcinoma. Int J Cancer 1980; 35: 85–9
van der Bruggen P, Traversari C, Chomez P, et al. A gene encoding an antigen recognized by cytolytic T lymphocytes on a human melanoma. Science 1991; 254: 1643–7
van der Bruggen P, van den Eynde B. Molecular definition of tumour antigens recognized by T lymphocytes. Curr Opin Immunol 1992; 4: 608–12
Traversari C, van der Bruggen P, Luescher JF, et al. A nonapeptide encoded by human gene MAGE-1 is recognized on HLA-A1 by cytotolytic T lymphocytes directed against tumour antigen MZ2-E. J Exp Med 1992; 176: 1453–7
Girling A, Bartkova J, Burchell J, et al. A core protein epitope of the polymorphic epithelial mucin detected by the monoclonal antibody SM-3 is selectively exposed in a range of primary carcinomas. Int J Cancer 1989; 43: 1072–6
Barnd DL, Lan M, Metzger R, et al. Specific, MHC-unrestricted recognition of tumour-associated mucins by human cytotoxic T cells. Proc Natl Acad Sci USA 1989; 86: 7159–63
Jerome KR, Barnd DL, Bendt KM, et al. Cytotoxic T-lymphocytes derived from patients with breast adenocarcinoma recognize an epitope present on the protein core of a mucin molecule preferentially expressed by malignant cells. Cancer Res 1991; 51: 2908–16
Hareuveni M, Gautier C, Kieny MP, et al. Vaccination against tumour cells expressing breast cancer epithelial tumour antigen. Proc Natl Acad Sci USA 1990; 87: 9498–502
Jung S, Schluesener HJ. Human T lymphocytes recognize a peptide of single point-mutated, oncogenic ras proteins. J Exp Med 1991; 173: 273–6
Prager MD, Gordon WC, Baechtel FS. Immunogenicity of modified tumour cells in syngeneic hosts. Ann NY Acad Sci 1976; 276: 61–74
Rosato FE, Brown AS, Miller EE, et al. Neuraminidase immunotherapy of tumours in man. Surg Gynecol Obstet 1974; 139: 675–82
Cassel WA, Murray DR, Phillips HS. A phase II study on the postsurgical management of Stage II malignant melanoma with a Newcastle disease virus oncolysate. Cancer 1983; 52: 856–60
Rapp HJ. A guinea pig model for tumour immunology: a summary. Israel J Med Sci 1973; 9: 366–74
Hunter JT, Okuda T, Rapp HJ. Immunotherapy of metastatic cancer in guinea pigs: failure of intralesional BCG to influence the results of radical surgery. J Natl Cancer Inst 1977; 59: 1435–9
Hanna Jr MG, Brandhorst JS, Peters LC. Active specific immunotherapy of residual micrometastases: an evaluation of sources, doses and ratios of BCG with tumour cells. Cancer Immunol Immunother 1979; 7: 165–73
Hoover Jr HC, Surdyke M, Dangel RB, et al. Delayed cutaneous hypersensitivity to autologous tumour cells in colorectal cancer patients immunized with an autologous tumour cell: bacillus Calmette-Guerin vaccine. Cancer Res 1984; 44: 1671–6
Mitchell MS, Kan-Mitchell J, Kempf RA, et al. Active specific immunotherapy for melanoma: phase I trial of allogeneic lysates and a novel adjuvant. Cancer Res 1988; 48: 5883–93
Livingston PO, Koganty R, Longenecker BM, et al. Studies on the immunogenicity of synthetic and natural Thomsen-Friedenreich (TF) antigens in mice: augmentation of the response by Quil A and SAF-m adjuvants and analysis of the specificity of the responses. Vaccine Res 1992; 1: 99–109
Newman MJ, Wu J-Y, Gardner BH, et al. Saponin adjuvant induction of ovalbumin-specific CD8+ cytotoxic T-lymphocyte responses. J Immunol 1992; 148: 2357–9
Fearon E, Pardoll D, Itaya T, et al. Interleukin-2 production by tumour cells bypasses T-helper function in the generation of an antitumour response. Cell 1990; 60: 397–403
Golumbek P, Lazenby A, Levitsky H, et al. Treatment of established renal cancer by tumour cells engineered to secrete interleukin-4. Science 1991; 254: 713–6
Gansbacher B, Bannerji G, Daniels B, et al. Retroviral vectormediated γ-interferon gene transfer into tumour cells generate potent and long lasting antitumour immunity. Cancer Res 1990; 50: 7820–5
Pardoll D. New strategies for active immunotherapy with genetically engineered tumour cells. Curr Opin Immunol 1992; 4: 619–23
Chen L, Ashe S, Brady WA, et al. Costimulation of antitumour immunity by the B7 counterreceptor for the T-lymphocyte molecules CD28 and CTLA-4. Cell 1992; 71: 1093–102
Townsand SE, Allison JP. Tumour rejection after direct costimulation of CD8+ T cells by B7-transfected melanoma cells. Science 1993; 259: 368–70
Azuma M, Phillips JH, Lanier LL. CD28 co-stimulation of Tcell-mediated cytotoxicity. Int J Cancer 1992; 7Suppl.: 33–5
Chen L, Linsley PS, Hellstrom KE. Stimulation of T cells for tumour immunity. Immunol Today 1993; 14: 483–6
Tartaglia J, Pinaes S, Pavletti E. Poxvirus-based vectors as vaccine candidates. Immunology 1990; 10: 13–30
Irvine K, Kantor J, Snoy P, et al. Characterization of immunological response using a CEA recombinant vaccinia virus in nonhuman primates. Proc Am Assoc Cancer Res 1992; 33: 334
Stover CK, De la Cuz VF, Fuerst TR, et al. New use of BCG for recombinant vaccines. Nature 1993; 351: 456–60
Hoover Jr HC, Brandhorst JS, Peters LC, et al. Adjuvant active specific immunotherapy for human colorectal cancer: 6.5-year median follow-up of a phase III prospectively randomized trial. J Clin Oncol 1993; 11: 390–9
Hoover Jr HC, Surdyke M, Dangel RB, et al. Prospectively randomized trial of adjuvant active-specific immunotherapy for human colorectal cancer. Cancer 1985; 55: 1236–43
Liebrich W, Schlag P, Manasterski M, et al. In vitro and clinical characterization of a Newcastle disease virus-modified autologous tumour cell vaccine for treatment of colorectal cancer patients. Eur J Cancer 1991; 27: 703–10
Lehner B, Schlag P, Liebrich W, et al. Postoperative active specific immunization in curatively resected colorectal cancer patients with a virus-modified autologous tumour cell vaccine. Cancer Immunol Immunother 1990; 32: 173–8
Manasterski M, Liebrich W, Bohle W, et al. Active specific immunotherapy in colorectal cancer and melanoma: comparison of DTH reaction with several characteristics of the vaccine. In: Klapdor R, editor. Recent results in tumour diagnosis and therapy. München, Bern, Wien, San Francisco: Zuckschwerdt, 1989: 153
Schlag P, Manasterski M, Gerneth T, et al. Active specific immunotherapy with Newcastle-disease-virus-modified autologous tumour cells following resection of liver metastases in colorectal cancer. Cancer Immunol Immunother 1992; 35: 325–30
MacLean GD, Bowen-Yacyshyn MB, Samuel J, et al. Active immunization of human ovarian cancer patients against a common carcinoma (Thomsen-Friedenreich) determinant using a synthetic carbohydrate antigen. J Immunother 1992; 11: 292–305
MacLean GD, Reddish M, Koganty RR, et al. Immunization of breast cancer patients using a synthetic sialyl-Tn glycoconjugate plus Detox adjuvant. Cancer Immunol Immunother 1993; 36: 215–20
Cohen J. Cancer vaccines get a shot in the arm. Science 1993; 262: 841–3
Mitchell MS, Harel W, Kan-Mitchell J, et al. Active specific immunotherapy of melanoma with allogeneic cell lysates: rationale, results, and possible mechanisms of action. Ann NY Acad Sci 1993; 690: 153–66
Wallack MK, Scoggin SD, Sivanandham M. Active specific immunotherapy with vaccinia melanoma oncolysate. Mt Sinai J Med 1992; 59: 227–33
Hersey P. Active immunotherapy with viral lysates of micro-metastases following surgical removal of high risk melanoma. World J Surg 1992; 16: 251–60
Higgins G. Antimelanoma vaccines may increase survival. Inpharma Weekly 1994; 934: 14–5
Berd D, Murphy G, Maguire Jr HC, et al. Immunization with haptenized, autologous tumour cells induces inflammation of human melanoma metastases. Cancer Res 1991; 51: 2731–4
Murphy G, Radu A, Kaminer M, et al. Autologous melanoma vaccine induces inflammatory responses in melanoma metastases: relevance to immunologic regression and immunotherapy. J Invest Dermatol 1993; 100: 335S–341S
Bystryn JC, Henn M, Shroba S. Identification of immunogenic human melanoma antigens in a polyvalent melanoma vaccine. Cancer Res 1992; 52: 5948–53
Bystryn JC, Oratz R, Roses D, et al. Relationship between immune response to melanoma vaccine immunization and clinical outcome in stage II malignant melanoma. Cancer 1992; 69: 1157–64
Euhus DM, Gupta RK, Morton DL. Induction of antibodies to a tumour-associated antigen by immunization with a whole melanoma cell vaccine. Cancer Immunol Immunother 1992; 29: 247–54
Livingston PO. Construction of cancer vaccines with carbohydrate and protein (peptide) tumour antigens. Curr Opin Immunol 1992; 4: 624–9
Woodbury RG, Brown JP, Yeh M-Y, et al. Identification of a cell surface protein, p79, in human melanomas and certain other neoplasms. Proc Natl Acad Sci USA 1980; 77: 2183–7
Furukawa KS, Furukawa R, Real FX, et al. Unique antigenic epitope of human melanoma is carried on the common melanoma glycoprotein Gp75/p97. J Exp Med 1989; 169: 585–90
Hellström KE, Hellstrom I. Oncogene-associated tumour antigens as targets for immunotherapy. FASEB J 1989; 3: 1715–22
Mittelman A, Chen ZJ, Yang H, et al. Human high molecular weight melanoma-associated antigen (HMW-MAA) mimicry by mouse anti-idiotypic monoclonal antibody MK2-23: induction of humoral anti-HMW-MAA immunity and prolongation of survival in patients with stage IV melanoma. Proc Natl Acad Sci USA 1992; 89: 466–70
Frodin JE, Faxas ME, Hagstrom B, et al. Induction of anti-idiotypic (ab2) and anti-anti-idiotypic (ab3) antibodies in patients treated with the mouse monoclonal antibody 17-1A (abi): relation to the clinical outcome - an important antitumoral effective function? Hybridoma 1991; 10: 421–31
Foon KA, Bhattacharya-Chatterjee M, Chakraborty M, et al. Murine anti-idiotype (Id) monoclonal antibody (mAb) induces specific humoral responses to carcinoembryonic antigen (CEA) in colorectal cancer (CRC) patients [abstract]. Proc Am Assoc Cancer Res 1994; 13: 294
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Schlag, P.M., Milleck, J. & Liebrich, W. Progress with Tumour Vaccines. Clin Immunother 2, 23–31 (1994). https://doi.org/10.1007/BF03258519
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF03258519