Summary
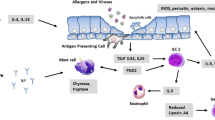
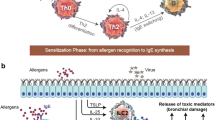
Many investigators now accept that chronic inflammation of the bronchial mucosa plays a fundamental role in the pathogenesis of asthma. This inflammation resembles a cell-mediated reaction in which selective release of cytokines, principally by activated T lymphocytes but also by other cells, brings about the accumulation and activation of granulocytes. Of the granulocytes, eosinophils are particularly implicated in causing the bronchial mucosal damage that is thought to underlie the clinical manifestations of asthma. Glucocorticoids, which inhibit T lymphocyte activation and cytokine release, currently form the mainstay of asthma therapy. However, many patients do not respond adequately to this therapy and consequently suffer from severe disease. Novel therapies for asthma, such as cytokine antagonists and monoclonal antibodies directed against pro-inflammatory effector cells, show great promise for the future. These agents are based on a knowledge of disease pathogenesis and are particularly directed against the T cell/cytokine/eosinophil interactions evident in the disease. They are urgently needed for those patients whose response to glucocorticoids is inadequate.
Similar content being viewed by others
References
Filley WV, Holley KE, Kephart GM, et al. Identification by immunofluorescence of eosinophil granule major basic protein in lung tissues of patients with bronchial asthma. Lancet 1982; 2: 11–2
Azzawi M, Bradley B, Jeffery PK, et al. Identification of activated T lymphocytes and eosinophils in bronchial biopsies in stable atopic asthma. Am Rev Respir Dis 1990; 142: 1410–3
Wardlaw AJ, Dunnette S, Gleich GJ, et al. Eosinophils and mast cells in bronchoalveolar lavage in mild asthma: relationship to bronchial hyperreactivity. Am Rev Respir Dis 1988; 137: 62–9
Jeffery PK, Wardlaw AJ, Nelson FC, et al. Bronchial biopsies in asthma: an ultrastructural, quantitative study and correlation with hyperreactivity. Am Rev Respir Dis 1989; 140: 1745–53
Bentley AM, Maestrelli P, Saetta M, et al. Activated T-lymphocytes and eosinophils in the bronchial mucosa in isocyanate-induced asthma. J Allergy Clin Immunol 1992a; 89: 821–9
Hamid Q, Barkans J, Robinson DS, et al. Co-expression of CD25 and CD3 in atopic allergy and asthma. Immunology 1992; 75: 659–63
Laitinen LA, Heino M, Laitinen A, et al. Damage of airway epithelium and bronchial reactivity in patients with asthma. Am Rev Respir Dis 1985; 131: 599–606
Bradley BL, Azzawi M, Assoufi B, et al. Eosinophils. T-lymphocytes, mast cells, neutrophils and macrophages in bronchial biopsies from atopic asthmatics: comparison with atopic non-asthma and normal controls and relationship to bronchial hyperresponsiveness. J Allergy Clin Immunol 1991; 88: 661–74
Fabbri LM, Danielli D, Crescioli S, et al. Fatal asthma in a subject sensitised to toluene diisocyanate. Am Rev Respir Dis 1988; 137: 1494–8
Bentley AM, Menz G, Storz C, et al. Identification of T-lymphocytes, macrophages and activated eosinophils in the bronchial mucosa in intrinsic asthma: relationship to symptoms and bronchial responsiveness. Am Rev Respir Dis 1992; 146: 500–6
Mattoli S, Mattoso VL, Soloperto M, et al. Cellular and biochemical characteristics of bronchoalveolar lavage fluid in symptomatic nonallergic asthma. J Allergy Clin Immunol 1991; 87: 794–802
Campbell HD, Tucker WQJ, Hort Y, et al. Molecular cloning. nucleotide sequence and expression of the gene encoding human eosinophil differentiation factor (interleukin-5). Proc Natl Acad Sci USA 1987; 84: 6629–33
Rothenberg ME, Owen WF, Silberstein DS, et al. Human eosinophils have prolonged survival, enhanced functional properties and become hypodense when exposed to human interleukin-3. J Clin Invest 1988; 81: 1986–92
Silberstein DS, Owen WF, Gasson JC, et al. Enhancement of human eosinophil cytotoxicity and leukotriene synthesis by biosynthetic (recombinant) granulocyte-macrophage colony stimulating factor. J Immunol 1986; 137: 3290–4
Basten A, Beeson PB. Mechanism of eosinophilia. iII. Role of the lymphocyte. J Exp Med 1970; 131: 1288–1305
Dent LA, Strath M, Mellor AL, et al. Eosinophilia in transgenic mice expressing interleukin-5. J Exp Med 1990; 172: 1425–31
Kameyoshi Y, Dorschner A, Mallet AI, et al. Cytokine RANTES released by thrombin-stimulated platelets is a potent attractant for human eosinophiles. J Exp Med 1992; 176: 587–92
Walsh GM, Hartneil A, Wardlaw AJ, et al. IL-5 enhances the in vitro adhesion of human eosinophils, but not neutrophils, in a leukocyte integrin (CD11/18) dependent manner. Immunology 1990; 71: 258–65
Walsh GM, Hartneil A, Mermod J-J, et al. Human eosinophil, but not neutrophil, adherence to IL-1 stimulated HUVEC is α4βl (VLA-4) dependent. J Immunol 1991; 146: 3419–23
Cromwell O, Wardlaw AJ, Champion A, et al. IgG-dependent generation of platelet-activating factor by normal and low density human eosinophils. J Immunol 1990; 145: 3862–8
Shaw RJ, Walsh GM, Cromwell O, et al. Activated human eosinophils generate SRS-A leukotrienes following physiological (IgG-dependent) stimulation. Nature 1985; 316: 150–2
Ayars GH, Altman LC, Gleich GJ, et al. Eosinophil and eosinophil granule mediated pneumocyte injury. J Allergy Clin Immunol 1985; 76: 595–604
Gleich GJ. The eosinophil and bronchial asthma: current understanding. J Allergy Clin Immunol 1990; 85: 422–36
Abu-Ghazaleh RI, Fujisawa T, Mestecky J, et al. IgA-induced eosinophil degranulation. J Immunol 1989; 142: 2393–400
Khaliffe J, Capron M, Cesbron JY, et al. Role of specific IgE antibodies in peroxidase (EPO) release from human eosinophils. J Immunol 1986; 137: 1659–64
Kroegel C, Yukawa T, Dent G, et al. Stimulation of degranulation from human eosinophils by platelet-activating factor. J Immunol 1989; 142: 3518–26
Braun RK, Franchini M, Erard F, et al. Human peripheral blood eosinophils produce and release interleukin-8 on stimulation with calcium ionophore. Eur J Immunol 1993; 23: 956–60
Del Pozo V, De Andres B, Martin E, et al. Murine eosinophils and IL-1: αIL-1 mRNA detection by in situ hybridization. J Immunol 1990; 144: 3117–22
Desreumaux P, Janin A, Colombel JF, et al. Interleukin 5 messenger RNA expression by eosinophils in the intestinal mucosa of patients with coeliac disease. J Exp Med 1992; 175: 293–7
Kita H, Ohnishi T, Okubo Y, et al. GM-CSF and interleukin-3 release from human peripheral blood eosinophils and neutrophils. J Exp Med 1991; 174: 745–8
Moqbel R, Hamid Q, Ying S, et al. Expression of mRNA and immunoreactivity for the granulocyte/macrophage colony stimulating factor (GM-CSF) in activated human eosinophils. J Exp Med 1991; 174: 749–52
Wong DT, Weller PF, Galli SJ, et al. Human eosinophils express transforming growth factor alpha. J Exp Med 1990; 172: 673–81
Azzawi M, Johnston PW, Majumdar S, et al. T lymphocytes and activated eosinophils in airway mucosa in fatal asthma and cystic fibrosis. Am Rev Respir Dis 1992; 145: 1477–82
Durham SR, Kay AB. Eosinophils, bronchial hyperreactivity and late-phase asthmatic reactions. Clin Allergy 1985; 15: 411–8
Bousquet J, Chanez P, Lacoste JY, et al. Eosinophilic inflammation in asthma. N Engl J Med 1990; 323: 1033–9
De Monchy JGR, Kauffman HF, Venge P, et al. Bronchoalveolar eosinophilia during allergen-induced late asthmatic reactions. Am Rev Respir Dis 1985; 131: 373–6
Diaz P, Gonzales MC, Galleguillos FR, et al. Leukocytes and lipid mediators in bronchoalveolar lavage during allergen-induced late-phase asthmatic reactions. Am Rev Respir Dis 1989; 139: 1383–9
Metzger WJ, Zavala D, Richerson HB, et al. Local allergen challenge and bronchoalveolar lavage of allergic asthmatic lungs: description of the model and local airway inflammation. Am Rev Respir Dis 1987; 135: 433–40
Lopez AF, Sanderson CJ, Gamble JR, et al. Recombinant human interleukin-5 is a selective activator of eosinophil function. J Exp Med 1988; 167: 219–24
Rothenberg ME, Petersen J, Stevens RL, et al. IL-5 dependent conversion of normodense human eosinophils to the hypodense phenotype uses 3T3 fibroblasts for enhanced viability, accelerated hypodensity and sustained antibody-dependent cytotoxicity. J Immunol 1989; 143: 2311–6
Burd PR, Rogers HW, Gordon JR, et al. Interleukin-3 dependent and independent mast cells stimulated with IgE and antigen express multiple cytokines. J Exp Med 1989; 170: 245–57
Cromwell O, Hamid Q, Corrigan CJ, et al. Expression and generation of IL-6, IL-8 and GM-CSF by human bronchial epithelial cells and enhancement by IL-Iβ and TNFα. Immunology 1992; 77: 330–7
Howell CJ, Pujol JL, Crea AE, et al. Identification of an alveolar macrophage-derived activity in bronchial asthma that enhances leukotriene C4 generation by human eosinophils stimulated by ionophore A23187 as a granulocyte-macrophage colony-stimulating factor. Am Rev Respir Dis 1989; 140: 1340–7
Corrigan CJ, Hartnell A, Kay AB. T-lymphocyte activation in acute severe asthma. Lancet 1988; 1: 1129–31
Corrigan CJ, Haczku A, Gemou-Engesaeth V, et al. CD4 T-lymphocyte activation in asthma is accompanied by increased serum concentrations of interleukin-5: effect of glucocorticoid therapy. Am Rev Respir Dis 1993; 147: 540–7
Corrigan CJ, Kay AB. CD4 T-lymphocyte activation in acute severe asthma. Relationship to disease severity and atopic status. Am Rev Respir Dis 1990; 141: 970–7
Walker C, Kaegi MK, Brown P, et al. Activated T cells and eosinophilia in bronchoalveolar lavages from subjects with asthma correlated with disease severity. J Allergy Clin Immunol 1991; 88: 935–42
Hamid Q, Azzawi M, Ying S, et al. Expression of mRNA for interleukin-5 in mucosal bronchial biopsies from asthma. J Clin Invest 1991; 87: 1541–6
Robinson DS, Hamid Q, Ying S, et al. Predominant Th2-type bronchoalveolar lavage T-lymphocyte population in atopic asthma. N Engl J Med 1992; 326: 298–304
Walker C, Virchow J-C, Bruijnzeel PLB, et al. T cell subsets and their soluble products regulate eosinophilia in allergic and nonallergic asthma. J Immunol 1991; 146: 1829–35
Flint KC, Leung KBP, Pearce FL, et al. Human mast cells recovered by bronchoalveolar lavage: their morphology, histamine release and the effects of sodium cromoglycate. Clin Sci 1985; 68: 427–32
Shanahan F, MacNiven I, Dyck N, et al. Human lung mast cells: distribution and abundance of histochemically distinct subpopulations. Int Arch Allergy Appl Immunol 1987; 83: 329–31
Schwartz LB, Irani AA, Roller K, et al. Quantitation of histamine, tryptase and chymase in dispersed human T and TC mast cells. J Immunol 1988; 139: 2724–9
Lawrence ID, Warner JA, Cohan VL, et al. Purification and characterization of human skin mast cells. Evidence for human mast cell heterogeneity. J Immunol 1987; 139: 3062–9
Peters SP, MacGlashan DW, Schulman ES, et al. Arachidonic acid metabolism in purified human lung mast cells. J Immunol 1984; 131: 1972–9
Irani AA, Craig SS, De Blois G, et al. Deficiency of tryptase-positive, chymase-negative mast cell type in gastrointestinal mucosa of patients with defective T-lymphocyte function. J Immunol 1987; 138: 4381–6
Flint KC, Leung KBP, Hudspith BN, et al. Bronchoalveolar mast cells in extrinsic asthma: a mechanism for the initiation of allergen-specific bronchoconstriction. BMJ 1985; 291: 923–6
Kirby JG, Hargreave FE, Gleich GJ, et al. Bronchoalveolar cell profiles of asthmatic and non-asthmatic subjects. Am Rev Respir Dis 1987; 136: 379–83
Tomioka M, Ida S, Yuriko S, et al. Mast cells in bronchoalveolar lumen of patients with bronchial asthma. Am Rev Respir Dis 1984; 129: 1000–5
Wardlaw AJ, Cromwell O, Celestino D, et al. Morphological and secretory properties of bronchoalveolar lavage mast cells in respiratory diseases. Clin Allergy 1986; 16: 163–73
Casale TB, Wood D, Richerson HB, et al. Elevated bronchoalveolar lavage fluid histamine levels in allergic asthmatics are associated with methacholine bronchial hyperreactivity. J Clin Invest 1987; 79: 1197–203
Diaz P, Galleguillos FR, Gonzalez MC, et al. Bronchoalveolar lavage in asthma: the effect of DSCG on leukocyte counts, immunoglobulins and complement. J Allergy Clin Immunol 1984; 74; 41–8
Howarth PH, Durham SR, Lee TH, et al. Influence of albuterol, cromolyn sodium and ipratropium bromide on the airway and circulating mediator responses to allergen bronchial provocation in asthma. Am Rev Respir Dis 1985; 132: 986–92
Murray JJ, Tonnel AB, Brash AR, et al. Release of prostaglandin D2 into human airways during acute antigen challenge. N Engl J Med 1986; 315: 800–4
Beasley R, Varley J, Robinson C, et al. Cholinergic-mediated bronchoconstriction induced by prostaglandin D2, its initial metabolite 9α, 11β-prostaglandin F2 and prostaglandin F2α in asthma. Am Rev Respir Dis 1987; 136: 1140–4
Holgate ST, Emanuel MB, Howarth PH. Astemizole and other H1 antihistamine drug treatment of asthma. J Allergy Clin Immunol 1985; 76: 375–82
Baeza ML, Reddigari SR, Haak-Frendscho M, et al. Purification and further characterisation of human mononuclear cell histamine releasing factor. J Clin Invest 1989; 83: 1204–12
Lee TH, Assoufi BK, Kay AB. The link between exercise, respiratory heat exchange and the mast cell in bronchial asthma. Lancet 1983; 1: 520–2
Bradding P, Feather IH, Howarth PH, et al. Interleukin-4 is localised to and released by human mast cells. J Exp Med 1992; 176: 1381–6
Schleimer RP. Effects of glucocorticosteroids on inflammatory cells relevant to their therapeutic applications in asthma. Am Rev Respir Dis 1990; 141: S59–S69
Corrigan CJ, Brown PH, Barnes NC, et al. Glucocorticoid resistance in chronic asthma: peripheral blood T-lymphocyte activation and a comparison of the T-lymphocyte inhibitory effects of glucocorticoids and cyclosporin A. Am Rev Respir Dis 1991; 144: 1026–32
Corrigan CJ, Brown PH, Barnes NC, et al. Glucocorticoid resistance in chronic asthma: glucocorticoid pharmacokinetics, glucocorticoid receptor characteristics and inhibition of peripheral blood T-cell proliferation by glucocorticoids in vitro. Am Rev Respir Dis 1991; 144: 1016–25
Robinson DS, Hamid Q, Ying S, et al. Prednisolone treatment in asthma is associated with modulation of bronchoalveolar lavage cell IL-4, IL-5 and IFN-gamma cytokine gene expression. Am Rev Respir Dis 1993; 148: 401–6
Richards IM, Shields SK, Griffin RL, et al. Novel steroid-based inhibitors of lung inflammation. Clin Exp Allergy 1992: 22: 432–9
Alexander AG, Barnes NC, Kay AB. Trial of cyclosporin in corticosteroid-dependent chronic severe asthma. Lancet 1992; 339: 324–8
Mihatsch MJ, Thiel G, Ryffel B. Hazards of cyclosporin A therapy and recommendations for its use. J Autoimmun 1988; 1: 533–43
Ledford DK. Alternative treatments for severe, chronic asthma. Allergy Proc 1993; 14: 23–30
Giembycz MA, Dent G. Prospects for selective cyclic nucleotide phosphodiesterase inhibitors in the treatment of bronchial asthma. Clin Exp Allergy 1992; 22: 337–44
Wegner CD, Gundel RH, Reilly P, et al. Intercellular adhesion molecule 1 (ICAM-1) in the pathogenesis of asthma. Science 1990; 247: 456–9
Cloud ML, Enas GC, Kemp J, et al. A specific LTD4/LTE4-receptor antagonist improves pulmonary function in patients with mild, chronic asthma. Am Rev Respir Dis 1989; 140: 1336–9
Powrie F, Coffman RL. Cytokine regulation of T-cell function: potential for therapeutic intervention. Immunol Today 1993: 14: 270–4
Bazan JG. Structural design and molecular evolution of a cytokine receptor superfamily. Proc Natl Acad Sci USA 1990: 87: 6934–8
Miyajima A, Kitamura T, Harada N, et al. Cytokine receptors and signal transduction. Annu Rev Immunol 1991; 10: 295–326
Winter G, Harris WJ. Humanized antibodies. Immunol Today 1993; 14: 243–6
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Corrigan, C.J. Immunological Aspects of Asthma. Clin. Immunother. 1, 31–42 (1994). https://doi.org/10.1007/BF03258489
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF03258489