Summary
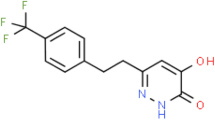
A comprehensive review of the extensive preclinical pharmacological data available on the pyrrolidinone derivative aniracetam has provided convincing evidence of ameliorative effects on learning and memory. Such effects were most clearly observed in animals exhibiting impaired cognitive abilities. Aniracetam was consistently shown to be considerably more potent than the structurally similar, prototypic nootropic piracetam, which was often included in these experiments as a parallel, active control compound. The mechanism of action of the nootropics, including aniracetam, has remained elusive, although it has been speculated that indirect facilitation of cholinergic neurotransmission, especially under conditions of dysfunction, may contribute. Recent studies indicate that aniracetam acts at the AMPA subtype of glutamate receptors as a positive modulator. Metabolites of aniracetam have been found to exhibit positive effects on memory and probably contribute to the cognition enhancement observed after aniracetam treatment. Finally, aniracetam was shown to be well tolerated under the diverse test conditions and in the numerous species evaluated. Thus, the cognition enhancer aniracetam exhibits a pharmacological profile suggesting considerable potential therapeutic value in the treatment of dementias.
Similar content being viewed by others
References
Alberici GP, Steinfels GF. Comparative effects of putative cognitive enhancing compounds. Abstract. Society for Neuroscience Abstracts 14: 249, 1988
Bandle EF, Wendt G, Ranalder UB, Trautmann K-H. 2-Pyrrolidinone and succinimide endogenously present in several mammalian species. Life Sciences 35: 2205–2212, 1984
Bartus RT, Dean RL, Beer B, Lippa AS. The cholinergic hypothesis of geriatric memory dysfunction. Science 217: 408–417, 1982
Bassant MH, Jobert A, Dutar P, Lamour Y. Effect of psychotropic drugs on identified septohippocampal neurons. Neuroscience 27: 911–920, 1988
Bering B, Mueller WE. Interaction of piracetam with several neurotransmitter receptors in the central nervous system. Arzneimittel Forschung 35: 1350–1352, 1985
Bowen DM, Francis PT. Neurochemistry, neuropharmacology and aetiological factors in Alzheimer’s disease. Seminars in Neuroscience 2: 101–108, 1990
Brugger F, Evans RH, Wicki U, Olpe HR, Pozza MF. The modulation of the quisqualate receptor by the nootropic drug aniracetam in the hemisected rat spinal cord preparation. European Journal of Neuroscience (Suppl. 4): 122, 1991
Cicardo VH, Carbone SE, Rondina DC, Mastronardi IO, Izquierdo JA. Effects of aniracetam on GABAergic-monoaminergic systems in rat brain and on motility: interactions with antagonists of dopamine receptors. International Research Communications System Medical Science 14: 1081–1082, 1986
Copani A, Genazzani AA, Aleppo G, Casabona G, Canonica P, et al. Nootropic drugs positively modulate alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid-sensitive glutamate receptors in neuronal cultures. Journal of Neurochemistry 58: 1199–1204, 1992
Cumin R, Bandle EF, Gamzu E, Haefely WE. Effects of the novel compound aniracetam (Ro 13-5057) upon impaired learning and memory in rodents. Psychopharmacology 78: 104–111, 1982
DeNoble VJ. Vinpocetine enhances retrieval of a step-through passive avoidance response in rats. Pharmacology, Biochemistry and Behavior 26: 183–186, 1987
DeNoble VJ, Repetti SJ, Gelpke LW, Wood LM, Keim KL. Vinpocetine: nootropic effects on scopolamine-induced and hypoxia-induced retrieval deficits of a step-through passive avoidance response in rats. Pharmacology, Biochemistry and Behavior 24: 1123–1128, 1986
Downs DA, Harrigan SE. Self-administration of Cl-844, hydergine and psychomotor stimulants by Rhesus monkeys. Abstract. International Journal of Neuroscience 32: 281, 1987
Flood JF, Cherkin A. Scopolamine effects on memory retention in mice: a model of dementia? Behavioral and Neural Biology 45: 169–184, 1986
Franceschi M, Alberoni M, Bressi S, Preda L, Cattaneo C, et al. Clinical models of phase I for the study of nootropic drugs. Pharmacological Research 22 (Suppl.): 214, 1990
Frankin SR, Sethy VH, Tang AH. Amnesia produced by intra-cerebroventricular injections of hemicholinium-3 in mice was prevented by pretreatment with piracetam-like compounds. Pharmacology, Biochemistry and Behavior 25: 925–927, 1986
Funk KF, Schmidt J. Zur cholinergen Wirkung von Nootropika. Biomedica Biochimica Acta 47: 417–421, 1988
Galliani G, Formento ML, Nencioni A, Barzaghi F. Animal models for screening nootropics. In Assandri A, Fumero S (Eds) Trends in drug development. Ivrea (Italy) Istituto di Ricierche Biomediche ‘A. Marxer’, pp. 43–74, 1989
Gamzu E, Boff E, Zolcinski M, Vincent G, Verderese T. A rapidly acquired, appetitively motivated, serial spatial discrimination reversal in rats for evaluating manipulations of learning and memory. Abstract. Society for Neuroscience Abstracts 9: 824, 1983
Gamzu E, Vincent G, Verderese A, Boff E, Lee L, et al. Pharmacological protection against memory retrieval deficits as a method of discovering new therapeutic agents. In Fisher A et al. (Eds) Alzheimer’s and Parkinson’s disease: strategies in research and development, pp. 375–391, Adv. Behav. Biol. Vol. 29, Plenum Press, New York, 1986
Genazzani AA, Copani A, Aleppo G, Canonico PL, Nicoletti F. Nootropic drugs as positive modulators of AMPA receptors. Abstract. Society for Neuroscience Abstracts 17: 796, 1991
Genkova MG, Lazarova MB. Influence of nootropic drugs on the learning- and memory-impairing effect of diethyldithiocarbamate in albino rats. Methods and Findings in Experimental and Clinical Pharmacology 10: 369–375, 1988
Genkova-Papasova M, Lazarova-Bakurova M. Influence of nootropic drugs on memory-impairing effect of diethyldithiocarbamate and clonidine in ‘step down’ passive avoidance in albino rats. Acta Physiologica Pharmacologica Bulgarica 14: 36–42, 1988
Giacobini E. Cholinergic receptors in human brain: effects of aging and Alzheimer’s disease. Journal of Neuroscience Research 27: 548–560, 1990
Gibson GE, Peterson C. Pharmacologic models of age-related deficits. In Crook T et al. (Eds) Assessment of geriatric psychopharmacology, pp. 323–343, Mark Powley Associates, New Canaan, Connecticut, 1983
Ginsberg MD, Busto R. Rodent models of cerebral ischemia. Stroke 20: 1627–1642, 1989
Giurgea C. The predictive value of animal experimentation for clinical geropsychopharmacology. Gerontology 32 (Suppl. 1): 37–45, 1986
Giurgea CE, Moyersoons FE. On the pharmacology of cortical evoked potentials. Archives Internationales de Pharmacodynamie et de Thérapie 199: 67–78, 1972
Gottfries CG. Pharmacological treatment strategies in dementia disorders. Pharmacopsychiatry 22: 129–134, 1989
Gottfries CG. Neurochemical aspects on aging and diseases with cognitive impairment. Journal of Neuroscience Research 27: 541–547, 1990
Haegele KD, Schwartz JJ, Schoun J, Schmitt AH, Schechter PJ. 2-Pyrrolidinone in human cerebrospinal fluid: a major constituent of total gamma-aminobutyric acid. Journal of Neurochemistry 49: 1402–1406, 1987
Hall ED, Von Voigtlander PF. Facilitatory effects of piracetam on excitability of motor nerve terminals and neuromuscular transmission. Neuropharmacology 26: 1573–1579, 1987
Himori N, Watanabe H, Matsuura A, Umeda Y, Kuwahara T, et al. General pharmacological properties of aniracetam, a cerebral insufficiency improver. Japanese Pharmacology and Therapeutics 14 (Suppl. 4): 93–128, 1986
Ikeda Y, Tanabe S, Takatoh M. Effect of dibenzoxazepine analogue (BY-1949) on acquisition process of operant behaviour in aged rats. Abstract. Psychopharmacology 96 (Suppl.): 305, 1988
Isaacson JS, Nicoll RA. Aniracetam reduces glutamate receptor desensitization and slows the decay of fast excitatory synaptic currents in the hippocampus. Proceedings of the National Academy of Sciences of the United States of America 88: 10936–10940, 1991
Ito I, Tanabe S, Kohda A, Sugiyama H. Allosteric potentiation of quisqualate receptors by a nootropic drug aniracetam. Journal of Physiology 424: 533–543, 1990
Itoh J, Nabeshima T, Kameyama T. Utility of an elevated plusmaze for the evaluation of memory in mice: effects of nootropics, scopolamine and electroconvulsive shock. Psychopharmacology 101: 27–33, 1990
Jaenicke B, Schulze G, Coper H. Motor performance achievements in rats of different ages. Experimental Gerontology 18: 393–407, 1983
Jaenicke B, Wrobel D, Schulze G. The effects of various drugs on the motor performance capacity of old rats. Pharmacopsychiatry 18: 136–137, 1985
Kaneko S, Sugimura M, Takahashi H, Inoue T, Satoh M. Selective inhibition of NMDA receptor function by nootropic drugs: elevation of the sites of action using mRNA-injected Xenopus oocyte responses and [3H]MK-801 binding assay. Abstract. Japanese Journal of Pharmacology 55 (Suppl.): 53P, 1991
King GA. Protection against hypoxia-induced lethality in mice. A comparison of the effects of hypothermia and drugs. Archvies Internationales de Pharmacodynamie et de Thérapie 286: 282–298, 1987
Kubota A, Furuya I, Kurasawa M. Pharmacological study of aniracetam II: choice reaction time-shortening action of aniracetam in aged rats; active metabolites, repeated treatment and sex difference. Japanese Pharmacology and Therapeutics 14 (Suppl. 4): 721–727, 1986a
Kubota A, Furuya I, Kurasawa M. Pharmacological study of aniracetam III: choice reaction time-shortening action of aniracetam in rat post-cerebrovascular disease deficiency model (SHR-SP). Japanese Pharmacology and Therapeutics 14 (Suppl. 4): 729–732, 1986b
Kubota A, Hayashi T, Sakagami T, Watanabe A, Nakamura K. Scopolamine model of retrograde amnesia: its prevention and relevant cerebral nuclei involved. In Saito S, Yanagita T (Eds) Learning and memory: drugs as reinforcers. pp. 96–118, Excerpta Medica, Amsterdam, 1982
Kubota A, Kurasawa M, Furuya I. Pharmacological study of aniracetam I: Choice reaction time-shortening action of aniracetam in aged rats; effective doses and reference drugs. Japanese Pharmacology and Therapeutics 14 (Suppl. 4): 713–720, 1986c
Kuwahara A, Kubota A, Hakkei M, Nakamura K. Drug dependence test on a cerebral insufficiency improver, aniracetam. Folia Pharmacologica Japonica 89: 33–46, 1987
Laurent J-P, Humbel U. Aniracetam-induced enhancement of synaptic transmission in rat hippocampal slices: extracellular analysis. Abstract. Neuroscience 22 (Suppl.): S349, 1987
Laurent J-P, Humbel U, Pozza M. Differential electrophysiological effects of aniracetam and piracetam in rat hippocampal slices. Presentation at IUPHAR 9th International Congress of Pharmacology, London, July 29–August 3, 1984
Lazarova-Bakarova M, Genkova-Papasova M. Effects of nootropic drugs on diethyldithiocarbamate- or clonidine-impaired learning and memory in rats. Abstract. Psychopharmacology 96 (Suppl.): S35, 1988
Lazarova-Bakarova MB, Genkova-Papasova MG. Influence of nootropic drugs on the memory-impairing effect of clonidine in albino rats. Methods and Findings in Experimental and Clinical Pharmacology 11: 235–239, 1989
Lorez H-P, Martin JR, Keller HH, Cumin R. Effect of aniracetam and the benzodiazepine receptor partial inverse agonist Ro 15-3505 on cerebral glucose utilization and cognitive function after lesioning of cholinergic forebrain nuclei in the rat. Drug Development Research 14: 359–362, 1988
Lundholm B, Sparf B. The effect of atropine on the turnover of acetycholine in the mouse brain. European Journal of Pharmacology 32: 287–292, 1975
Martin JR, Cumin R, Aschwanden W, Moreau J-L, Jenck F, et al. Aniracetam improves maze performance in rats. Neuro-Report 3: 81–83, 1992
Matsuyama K, Miyazaki C, Masataka I. Development of a pyrrolidinone derivative (cyclic GABA) for modulating brain glutamate tranmission. Advances in Behavioral Biology 36: 185–196, 1989
Miyazaki C, Matsuyama K, Ichikawa M, Goto S, Yamamoto J. Synthesis of valproyl-2-pyrrolidinone and its evaluation as a cognitive drug with the ability to modulate acidic amino acids in the brain. Journal of Pharmacobio-Dynamics 13: 70–75, 1990
Mizuki Y, Yamada M, Katoh I, Takada Y, Tsujimaru S, et al. Effects of aniracetam, a nootropic drug, in senile dementia. Kurume Medical Journal 31: 135–143, 1984
Mondadori C, Bhatnagar A, Borkowski J, Haeusler A. Involvement of a steroidal component in the mechanism of action of piracetam-like nootropics. Brain Research 506: 101–108, 1990
Mondadori C, Ducret T, Borkowski J. The memory enhancing effects of the piracetam-like nootropics are dependent on experimental parameters. Behavioural Brain Research 33: 79–82, 1989a
Mondadori C, Ducret T, Borkowski J. How long does ‘memory consolidation’ take? New compounds can improve retention performance, even if administered up to 24 hours after the learning experience. Brain Research 555: 107–111, 1991
Mondadori C, Ducret T, Petschke F. Blockade of the nootropic action of piracetam-like nootropics by adrenalectomy: an effect of dosage? Behavioural Brain Research 34: 155–158, 1989b
Mondadori C, Petschke F. Do piracetam-like compounds act centrally via peripheral mechanism? Brain Research 435: 310–314, 1987
Moreau J-L, Jenck F, Bonetti EP, Martin JR, Haefely WE. Novel long-acting benzodiazepine receptor ligands Ro 41-7812 and Ro 42-8773: neurological and behavioral profile. Drug Development Research 22: 375–383, 1991
Nabeshima T, Noda Y, Tohyama K, Itoh J, Kameyama T. Effects of DM-9384 in a model of amnesia based on animals with GABAergic neuronal dysfunctions. European Journal of Pharmacology 178: 143–149, 1990a
Nabeshima T, Ogawa S, Kameyama T, Shiotani T, Takasu Y, et al. Effects of DM-9384 and aniracetam on learning in normal and basal forebrain-lesioned rats. Research Communications in Psychology, Psychiatry and Behavior 16: 1–14, 1991a
Nabeshima T, Tohyama K, Kameyama T. Effects DM-9384, a pyrrolidinone derivative, on alcohol- and chloridiazepoxide-induced amnesia in mice. Pharmacology, Biochemistry and Behavior 36: 233–236, 1990b
Nabeshima T, Tohyama K, Murase K, Ishihara S, Kameyama T, et al. Effects of DM-9384, a cyclic derivative of GABA, on amnesia and decreases in GABAa and muscarinic receptors induced by cycloheximide. Journal of Pharmacology and Experimental Therapeutics 257: 271–275, 1991b
Nakajima T, Kubota A, Nakamura K. Anti-amnestic agent and post-proline cleaving enzyme inhibition. Abstract. Folia Phar-macologica Japonica 82: 154P–155P, 1983
Nakajima T, Takahashi M, Okada T. Pharmacological study of aniracetam VI: effects of aniracetam on muscarinic acetylcholine receptors in the rat hippocampus. Japanese Pharmacology and Therapeutics 14 (Suppl. 4): 751–757, 1986
Nakayama S, Ichihara S, Ichihara Y, Sakata H, Tomisawa H, et al. Pharmacokinetic study on aniracetam in rats I: Blood level profile, tissue distribution, and excretion after a single oral administration. Japanese Pharmacology and Therapeutics 14 (Suppl. 4): 129–145, 1986
Ohno M, Yamamoto T, Kitajima I, Ueki S. WEB 1881 FU ameliorates impairment of working memory induced by scopolamine and cerebral ischemia in the 3-panel runway task. Abstract. Japanese Journal of Pharmacology 49 (Suppl.): 267P, 1989
Okada T, Satoh T, Yajima T. Pharmacological study of aniracetam IV: a protective effect of aniracetam on decrease in deoxyglucose uptake in the rat brain under anemic hypoxia. Japanese Pharmacology and Therapeutics 14 (Suppl. 4): 733–740, 1986
Okuyama S, Aihara H. Action of nootropic drugs on transcallosal responses in rats. Neuropharmacology 27: 67–72, 1988
Ozawa S, Lino M, Abe M. Excitatory synapse in the rat hippocampus in tissue culture and effects of aniracetam. Neuroscience Research 12: 72–82, 1991
Parnetti L, Bartorelli L, Bonaiuto S, Cucinotta D, Cuzzupoli M, et al. Aniracetam (Ro 13-5057) for the treatment of senile dementia of Alzheimer type: results of a multicentre clinical study. Dementia 2: 262–267, 1991
Pepeu G, Marconcini Pepeu I, Casamenti F. The validity of animal models in the search of drugs for the aging brain. Drug Design and Delivery 7: 1–10, 1990
Perio A, Terranova JP, Worms P, Bluthe RM, Dantzer R, et al. Specific modulation of social memory in rats by cholinomimetic and nootropic drugs, by benzodiazepine inverse agonists, but not by psychostimulants. Psychopharmacology 97: 262–268, 1989
Petkov VD, Getova D, Mosharrof AH. A study of nootropic drugs for anti-anxiety action. Acta Physiologica et Pharmacologica Bulgaria 13: 25–30, 1987
Petkov VD, Grahovska T, Petkov VV, Konstantinova E, Stancheva S. Changes in the brain biogenic monoamines of rats induced by piracetam and aniracetam. Acta Physiologica et Pharmacologica Bulgarica 10: 6–14, 1984
Petkov VD, Kehayov R. Individually-determined differences in the effects of psychotropic drugs on memory (experiments on rats). Acta Physiologica et Pharmacologica Bulgarica 13: 30–36, 1987
Petkov VD, Mosharrof AH, Milenkov B, Enev V. Learning and memory effects of four newly-synthesized aniracetam analogues. Acta Physiologica et Pharmacologica Bulgarica 15: 18–26, 1989
Petkov VD, Mosharrof AH, Petkov VV, Kehayov RA. Age-related differences in memory and in the memory effects of nootropic drugs. Acta Physiologica et Pharmacologica Bulgarica 16: 28–36, 1990
Pieri L, Schaffner R, Scherschlicht R, Polc P, Sepinwall J, et al. Pharmacology of midazolam. Arzneimittel-Forschung 31: 2180–2201, 1981
Pietrusiak N, Vincent GP, Harney R, Sepinwall J. Use of proximal vs. distal cues in a water maze task is strain dependent: Aniracetam (Ro 13-5057) reverses distal cue deficits. Abstract. Society for Neuroscience Abstracts 12: 705, 1986
Pizzi M, Fallacara C, Memo M, Spano PF. Aniracetam prevents glutamate-induced neurotoxicity. Abstract. Society for Neuroscience Abstracts 17: 792, 1991
Pontecorvo MJ, Evans HL. Effects of aniracetam on delayed matching-to-sample performance on monkeys and pigeons. Pharmacology, Biochemistry and Behavior 22: 745–752, 1985
Poschel BPH, Ho PM, Ninteman FW, Callahan MJ. Pharmacologic therapeutic window of pramiracetam demonstrated in behavior, EEG, and single neuron firing rates. Experientia 41: 1153–1156, 1985
Poschel BPH, Marriott JG, Gluckman MI. Pharmacology of the cognition activator pramiracetam (CI-879). Drugs Under Experimental and Clinical Research 9: 853–871, 1983
Pozzi O, Allievi E, Biagetti R, Banfi S, Dorigotti L. Behavioural screening for potential nootropic drugs. Abstract. Pharmacological Research Communications 20 (Suppl. 2): 315, 1988
Rigter H, Martinez JL, Crabbe JC. Forgetting and other behavioral manifestations of aging. In Stein DG (Ed.) The psychobiology of aging, pp. 161–175, Elsevier/North Holland, Amsterdam, 1980
Roca J, Balasch J. Effect of nicardipine on vertebral blood flow in dogs. Drugs Under Experimental and Clinical Research 10: 399–403, 1984
Rousseva S, Petkov VV, Petkov VD, Voronina TA, Nerobkova LN, et al. Memory effects of the combination of medazepam with nootropic agents. Acta Physiologica et Pharmacologica Bulgarica 14: 27–35, 1988
Rumennik L, Vincent GP, Schwam E, Sepinwall J. A comparison of drug-induced effects on acquisition and retention in the squirrel monkey. Abstract. Society for Neuroscience Abstracts 14: 60, 1988
Sakurai T, Hatanaka S, Tanaka S, Yamasaki T, Kojima H, et al. Protective effect of DM-9384, a novel pyrrolidinone derivative, against experimental cerebral anoxia. Japanese Journal of Pharmacology 54: 33–43, 1990
Sakurai T, Ojima H, Yamasaki T, Kojima H, Akashi A. Effects of N-(2,6-dimethylphenyl)-2-(2-oxo-1-pyrrolidinyl) acetamide (DM-9384) on learning and memory in rats. Japanese Journal of Pharmacology 50: 47–53, 1989
Saletu B, Gruenberger J. The hypoxia model in human psychopharmacology: neurophysiological and psychometric studies with aniracetam i.v. Human Neurobiology 3: 171–181, 1984
Saletu B, Gruenberger J, Linzmayer L. Quantitative EEG and psychometric analyses in assessing CNS-activity of Ro 13-5057 — a cerebral insufficiency improver. Methods and Findings in Experimental and Clinical Pharmacology 2: 269–285, 1980
Santucci V, Fournier M, Worms P, Keane P, Bizière K. Cerebralactivating (EEG) properties of two inverse agonists and of an antagonist at the benzodiazepine receptor in the rat. Naunyn-Schmiedeberg’s Archives of Pharmacology 340: 93–100, 1989
Satoh M, Ishihara K, Iwama T, Takagi H. Aniracetam augments, and midazolam inhibits, the long-term potentiation in guineapig hippocampal slices. Neuroscience Letters 68: 216–220, 1986
Satoh M, Ishihara K, Katsuki H. A pharmacological profile of LTP in CA3 region of guinea-pig hippocampus in vitro. Biomedical Research 10 (Suppl. 2): 125–129, 1989
Schindler U, Rush DK, Fielding S. Nootropic drugs: animal models for studying effects on cognition. Drug Development Research 4: 567–576, 1984
Schwam E, Keim K, Cumin R, Gamzu E, Sepinwall J. The effects of aniracetam on primate behavior and EEG. Annals of the New York Academy of Sciences 444: 482–484, 1985
Senin U, Abate G, Fieschi C, Gori G, Guala A, et al. Aniracetam (Ro 13-5057) in the treatment of senile dementia of Alzheimer type (SDAT): results of a placebo controlled multicentre clinical study. European Neuropsychopharmacology 1: 511–517, 1991
Sethy VH. Effect of piracetam on high affinity choline uptake. Abstract. Society for Neuroscience Abstracts 9: 429, 1983
Shih YH, Pugsley TA. The effects of various cognition-enhancing drugs on in vitro rat hippocampal synaptosomal sodium dependent high affinity choline uptake. Life Sciences 36: 2145–2152, 1985
Smith G. Animal models of Alzheimer’s disease: experimental cholinergic denervation. Brain Research Reviews 13: 103–118, 1988
Spignoli G, Pepeu G. Interactions between oxiracetam, aniracetam and scopolamine on behavior and brain acetylcholine. Pharmacology, Biochemistry and Behavior 27: 491–495, 1987
Spirt NM, Mangano RM. Modulation of ligand binding to muscarinic cholinergic receptors. Abstract. Society for Neuroscience Abstracts 12: 993, 1986
Stancheva SL, Alova LG. Effects of centrophenoxine, piracetam and aniracetam on monoamine oxidase activity in different rat brain structures. (Russian with English abstract). Famakologiia i Toksikologiia 51: 16–18, 1988
Staubli U, Kessler M, Lynch G. Aniracetam has proportionately smaller effects on synapses expressing long-term potentiation: Evidence that receptor changes subserve LTP. Psychobiology 18: 377–381, 1990
Tang C-M, Shi Q-Y, Katchman A, Lynch G. Modulation of the time course of fast EPSCs and glutamate channel kinetics by aniracetam. Science 254: 288–290, 1991
Tobe A, Yamaguchi T, Nagai R, Egawa M. Effects of bifemelane hydrochloride (MCI-2016) on experimental amnesia (passive avoidance failure) in rodents. Japanese Journal of Pharmacology 39: 153–161, 1985
Todorov S, Zamfirova R, Petkov VD. Study of the effects of nootropic agents on the adrenergic neurotransmission in smooth muscles of young and old animals. Acta Physiologica Pharmacologica Bulgarica 16: 38–45, 1990
Tohyama K, Nabeshima T, Kameyama T. Effect of DM-9384, a pyrrolidinone derivative, on benzodiazepine-induced amnesia. Abstract. Psychopharmacology 96 (Suppl.): 305, 1988
Toide K. Effects of aniracetam on one-trial passive avoidance tests and cholinergic neurons in discrete brain regions of rats. Archives Internationales de Pharmacodynamie et de Thérapie 298: 25–37, 1989
Tsuzuki K, Takeuchi T, Ozawa S. Agonist- and subunit-dependent potentiation of glutamate receptors by a nootropic drug aniracetam. Molecular Brain Research 16: 105–110, 1992
Verderese A, Vincent GP, Fernia P, Sepinwall J. The effects of aniracetam (Ro 13-5057) on memory consolidation and reversal of retrieval deficits in C57BL/6 and C57BL/10 mice. Abstract. Society for Neuroscience Abstracts 12: 705, 1986
Vincent G, Verderese A, Gamzu E. The effects of aniracetam (Ro 13-5057) and piracetam on the enhancement of memory in mice. Abstract. Society for Neuroscience Abstracts 10: 258, 1984
Vincent G, Verderese A, Gamzu E. The effects of aniracetam (Ro 13-5057) on the enhancement and protection of memory. Annals of the New York Academy of Sciences 444: 489–491, 1985
Vincent GP, Sepinwall J. The memory enhancing effects of aniracetam (Ro 13-5057) on spatial memory are strain dependent. Abstract: Society for Neuroscience Abstracts 12: 704, 1986
Vyklicky L, Patneau DK, Mayer ML. Modulation of excitatory synaptic transmission by drugs that reduce desensitization at AMPA/kainate receptors. Neuron 7: 971–984, 1991
Watabe S, Yamaguchi H, Ashida S. effects of DM-9384, a new cognition-enhancing agent, on cholinergic system in rat cortex. Abstract. Society for Neuroscience Abstracts 16: 137, 1990
Watabe S, Yoshii M, Yamaguchi H, Ashida S. DM-9384, a new cognition-enhancing agent, is a potent facilitator of neuronal Ca channel activity as compared with other pyrrolidone derivatives. Abstract. Society for Neuroscience Abstracts 17: 63, 1991
Wesnes K, Anand R, Simpson P, Christmas L. The use of a scopolamine model to study the potential nootropic effects of aniracetam and piracetam in healthy volunteers. Journal of Psychopharmacology 4: 219–232, 1990
Whitehouse P, Price D, Struble R, Clark A, Coyle J, et al. Alzheimer’s disease and senile dementia: loss of neurons in basal forebrain. Science 215: 1237–1239, 1983
Worms P, Bizière K. Antagonism by cholinomimetic drugs of the turning induced by intrastriatal pirenzepine in mice. Psychopharmacology 93: 489–493, 1987
Worms P, Kan J-P, Steinberg R, Terranova J-P, Perio A, et al. Cholinomimetic activities of minaprine. Naunyn-Schmiedeberg’s Archives of Pharmacology 340: 411–418, 1989
Xiao P, Staubli U, Kessler M, Lynch G. Selective effects of aniracetam across receptor types and forms of synaptic facilitation in hippocampus. Hippocampus 1: 373–380, 1991
Yamada K, Inoue T, Tanaka M, Furukawa T. Prolongation of latencies for passive avoidance responses in rats treated with aniracetam or piracetam. Pharmacology, Biochemistry and Behavior 22: 645–648, 1985
Yamamoto J, Toide K, Kasahara N, Matsuura N, Matsushita Y. Effects of various cerebroactive drugs on EEG activity in rabbits and anoxia in mice. Abstract. Japanese Journal of Pharmacology 46 (Suppl.): 167P, 1988
Yoshimoto T, Kado K, Matsubara F, Koriyama N, Kaneto H, Tsuru D. Specific inhibitors for prolyl endopeptidase and their anti-amnesic effect. Journal of Pharmacobio-Dynamics 10: 730–735, 1987
Yoshizaki H, Okada T. Pharmacological study of aniracetam V: inhibitory effect of aniracetam on scopolamine induced increase of sodium dependent high affinity choline uptake into rat hippocampus. Japanese Pharmacology and Therapeutics 14 (Suppl. 4): 741–750, 1986
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Martin, J.R., Haefely, W.E. Pharmacology of Aniracetam. Drug Invest 5 (Suppl 1), 4–49 (1993). https://doi.org/10.1007/BF03258426
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF03258426