Summary
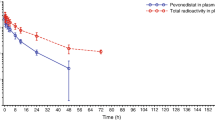
The pharmacokinetics of perrimustine and cystemustine, 2 novel 2-chloroethylnitrosoureas (CENUs), were investigated in patients with various cancer diseases. The unique protocol followed in this study consisted of a 15-minute intravenous (IV) perfusion of a 45 mg/m2 dose of perrimustine every 4 weeks and a 60 or 90 mg/m2 dose of cystemustine every 2 weeks. A sensitive and specific method was developed using solid phase extraction and HPLC analysis, which allowed measurement of the drug concentration levels until at least 3 hours after the beginning of administration of the injection. The rate of disappearance of the 2 drugs from the blood was fitted to either a monoexponential or a biexponential model. When detected, the half-lives of the distribution phase were less than 10 minutes. Regardless of the pharmacokinetic model used, the elimination half-lives were about 50 minutes. The interindividual variations of the pharmacokinetic parameters, as reflected by the variation coefficients (10 to 47%), were lower than those reported in the literature for this class of unstable anticancer drugs. The procedure described here allows one to safely conduct perrimustine and cystemustine pharmacokinetic studies with the purpose of further investigation in a greater number of patients to determine whether any correlation exists between pharmacokinetics, efficacy and/or toxicity.
Similar content being viewed by others
References
Bourut C, Chenu E, Godenèche D, Madelmont JC, et al. Cytostatic action of two nitrosoureas derived from cysteamine. British Journal of Cancer 89: 539–546, 1986
Filippeschi S, Colombo T, Bassani D, de Francesco L, et al. Antitumor activity of the novel nitrosourea S 10036 in rodent tumors. Anticancer Research 8: 1351–1354, 1988
Godenèche D, Labarre P, Moreau MF, Madelmont JC, et al. Main urinary metabolites of two cysteamine containing 2-(chloroethyl) nitrosoureas in rats. Drug Metabolism and Disposition 21: 91–99, 1993
Godenèche D, Madelmont JC, Labarre P, Plagne R, Meyniel G. Disposition of new sulphur-containing 2-(chloroethyl) nitrosoureas in rats. Xenobiotica 17: 59–70, 1987
Gunnarsson PO, Vibe-Petersen J, Macpherson JS, Warrington PS, et al. Pharmacokinetics of tauromustine in cancer patients. Cancer Chemotherapy and Pharmacology 23: 176–180, 1989
Hansch C, Smith N, Engle R, Wood H. Quantitative structure-activity relationships of antineoplastic drugs: nitrosoureas and triazenoimidazoles. Cancer Chemotherapy Reports 56: 443–456, 1972
Kim-Triana B, Misset JL, Madelmont JC, Godenèche D, et al. Phase I trial of perrimustine, a new cysteamine (2-chloroethyl) nitrosourea: an intrapatient escalation scheme. Anticancer Drugs 3: 225–231, 1992
Labarre P, Godenèche D, Madelmont JC, Kim-Triana B, et al. Qualitative and quantitative analysis of a new sulfur containing nitrosourea in blood by high performance liquid chromatography. Journal of Chromatography 419: 381–387, 1987
Lokiec F, Beerblock K, Deloffre P, Lucas C, Bizzari JP. Etude pharmacocinétique clinique de la fotémustine dans différentes indications tumorales. Bulletin du Cancer 76: 1063–1069, 1989
Lucas C, Ings B, Gray AJ, Deloffre P, et al. Comparison inter-espèce des paramètres pharmacocinétiques de la fotémustine (Nitrosourée S 10036): souris, rat, singe, chien et homme. Bulletin du Cancer 76: 863–865, 1989
Madelmont JC, Godenèche D, Parry D, Duprat J, et al. New cysteamine (2-chloroethyl) nitrosoureas. Synthesis and preliminary antitumor results. Journal of Medicinal Chemistry 28: 1346–1350, 1985
Mathé G, Misset JL, Kim-Triana B, Godenèche D, et al. Phase I trial of cystemustine, a new cysteamine (2-chloroethyl) nitrosourea: an intrapatient escalation scheme. Drugs Under Experimental and Clinical Research 18: 155–158, 1992
Schabel Jr FM. Nitrosoureas: a review of experimental antitumor activity. Cancer Treatment Reviews 9: 665–698, 1976
Weiss RB, Issell BF. The nitrosoureas carmustine (BCNU) and lomustine (CCNU). Cancer Treatment Reviews 9: 313–330, 1982
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Godenèche, D., Labarre, P., Cussac, C. et al. Pharmacokinetics of Two New 2-Chloroethylnitrosoureas in Cancer Patients Submitted to Phase II Clinical Trials. Drug Invest 7, 234–243 (1994). https://doi.org/10.1007/BF03257415
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF03257415