Abstract
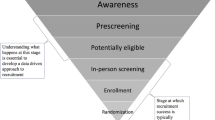
Recruitment for clinical trials is challenging for any disease but is particularly so for Alzheimer’s disease (AD). In the past three years, our site has recruited almost 600 subjects into our AD primary prevention and treatment trials, and diagnostic studies. In this article, we describe which strategies have had the best yield and which have had a modest or low yield.
High yield is loosely defined as any recruitment activity that yields a high number of interested or eligible participants per the expense or time/staff invested in the recruitment effort. For example, a high-yield recruitment strategy is direct mailings for primary prevention trials. Low yield is defined as any recruitment activity that yields a low number of interested or eligible participants per the expense or time/staff invested in the recruitment effort. For example, direct mailing to physicians generally does not yield many subjects. Recruitment for primary prevention trials and treatment trials differ in approach.
Recruitment of subjects is much more expensive than previously reported in the literature. It is important to build this expense into budgets. Subjects that do not qualify for their desired study may be candidates for other studies. Minority recruitment presents unique challenges above and beyond the overall challenges of successful recruitment in to AD clinical trials.



Similar content being viewed by others
References
Patrick JH, Pruchno RA, Rose MS. Recruiting research participants: a comparison of costs and effectiveness of five recruitment strategies. Gerontologist 1998; 38(2): 295–302
Cohen-Mansfield J. Recruitment rates in gerontological research: the situation for drug trials in dementia may be worse than previously reported. Alzheimer Dis Assoc Disord 2002; 16(4): 279–82
Thal LJ. Potential prevention strategies for Alzheimer disease. Alzheimer Dis Assoc Disord 1996 Fall; 10Suppl. 1: 6–8
Cosgrove N, Borhani NO, Bailey G, et al. Mass mailing and staff experience in a total recruitment program for a clinical trial: the SHEP experience. Control Clin Trials 1995; 19: 133–48
Kuller L. Panel discussion. In: Wenger NK, editor. Inclusion of elderly in clinical trials: cardiovascular therapy as a model. Knsas City (MO): Marion Merrell Dow, 1993: 83–5
Boult C, Boult L, Morishita L, et al. Soliciting defined populations to recruit samples of high-risk older adults. J Gerontol 1998; 53(5): M379–84
Ives DG, Kuller LH, Schulz R, et al. Comparison of recruitment strategies and associated disease prevalence for health promotion in rural elderly. Prev Med 1992; 21: 582–91
Taylor-Davis S, Smiciklas-Wright H, Davis AC, et al. Time and cost for recruiting older adults. J Am Geriatr Soc 1998; 46: 753–7
Marx MS, Cohen-Mansfield J, Guralnik JM, et al. Recruiting community-dwelling elderly at risk for physical disability into exercise research. J Aging Physical Ability 2003; 11(2): 229–41
National Institute on Aging (NIA)/University of Washington. Alzheimer’s Disease Anti-inflammatory Prevention Trial (ADAPT), U01AG15477. Sattle (WA): University of Washington, 2000. (Data on file)
Tarlow BA, Mahoney DF. The cost of recruiting Alzheimer’s disease caregivers for research. J Aging Health 2000; 12(4): 490–510
Gill TM, McGloin JM, Gahbauer EA, et al. Two recruitment strategies for a clinical trial of physically frail community-living older persons. J Am Geriatr Soc 2001; 49(8): 1039–45
Adams J, Sliverman M, Musa D, et al. Recruiting older adults for clinical trials. Control Clin Trials 1997; 18: 14–26
Sparks DL, Sabbagh MN, Breitner JCS, et al. Is cholesterol a culprit in Alzheimer’s disease? Int Psychogeriatr 2003; 15Suppl. 1: 153–9
Treves TA, Verchovsky R, Klimovitsky S, et al. Recruitment rate to drug trials for dementia of the Alzheimer type. Alzheimer Dis Assoc Disord 2000; 14(4): 209–11
Levy MI, Mohs RC, Rosen WG, et al. Research subject recruitment for gerontological studies of pharmacological agents. Neurobiol Aging 1982; 3(1): 77–9
Williams DE, Vitiello MV, Ries RK, et al. Successful recruitment of elderly community-dwelling subjects for Alzheimer’s disease research. J Gerontol 1988; 43(3): M69–74
Anderson LA, Fogler J, Dedrick RF, et al. Recruiting from the community: lessons learned from the Diabetes Care for Older Adults project. Gerontologist 1995; 35(3): 395–401.
Hall WD. Screening and recruitment of elderly participants into large-scale cardiovascular research trials. In: Wenger NK, editor. Inclusion of elderly in clinical trials: cardiovascular disease and cardiovascular therapy as a model. Knsas City (MO): Marion Merrell Dow, 1993: 67–71
Ballard EL, Nash F, Raiford K, et al. Recruitment of black elderly for clinical research studies of dementia: the CERAD experience. Gerontologist 1993; 33(4): 561–5
Connell CM, Shaw BA, Holmes SB, et al. Caregivers’ attitudes toward their family members’ participation in Alzheimer disease research: implications for recruitment and retention. Alzheimer Dis Assoc Disord 2001; 15(3): 137–45
Wrobel AJ, Shapiro NE. Conducting research with urban elders: issues of recruitment, data collection, and home visits. Alzheimer Dis Assoc Disord 1999; 13Suppl. 1: S34–8
Dowling GA, Wiener CL. Roadblocks encountered in recruiting patients for a study of sleep disruption in Alzheimer’s disease. Image J Nurs Sch 1997; 29(1): 59–64
Korczyn AD. Recruitment for drug studies in dementia. Alzheimer Dis Assoc Disord 2002; 16(4): 283–4
Warren JW, Sobal J, Tenney JH, et al. Informed consent by proxy: an issue in research with elderly patients. New Engl J Med 1986; 315(18): 1124–8
Mastwyk M, Ritchie CW, LoGiudice D, et al. Caregiver impressions of participation in Alzheimer’s disease clinical trials: what are their hopes? And is it worth it? Int Psychogeriatr 2002; 14(1): 39–45
Acknowledgements
We wish to thank Bonnie Tigner for her technical assistance in the preparation of this manuscript. Furthermore, we wish to thank Dr John Morris of Washington University and Dr John Breitner of the University of Washington for their editorial contributions. We wish to thank Jacqueline Bochenek of the Alzheimer’s Disease Cooperative Study at the University of California-San Diego for some of the information provided (US-NIA-AG10483). This study was funded by: US-NIA-AG019610. There are no conflicts of interest to report for any named author pertaining to this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sabbagh, M.N., Thompson, N., Tweedy, D. et al. Recruitment and Retention Strategies for Clinical Trials in Alzheimer’s Disease. Pharm Dev Regul 1, 269–276 (2003). https://doi.org/10.1007/BF03257386
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF03257386