Abstract
This paper presents state-of-the-art statistical methods for dose-finding experiments in first-in-man clinical studies of drugs for the treatment of malignancies. Most early-phase clinical trials are not hypothesis driven, which might be the reason why statistical considerations have been largely ignored in dose-finding studies. The standard experimental design for dose-finding clinical studies employs a rule-based, dose-escalation scheme in which escalation depends on the number of patients at a dose level who experience dose-limiting toxicity. The standard design is widely used because of its algorithm-based simplicity for clinical investigators.
In the last two decades, new approaches for dose-finding have been proposed, all aiming to (i) model the toxicity of a new treatment as a percentile of the dose-toxicity relationship; (ii) minimize the number of patients treated at unacceptably high toxic dose levels; and (iii) minimize the number of patients needed to complete the study. In this paper, we describe some of these methodologies in simple terms for nonstatisticians.


Similar content being viewed by others
Notes
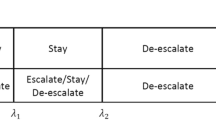
A gain function is the mathematical representation in Bayesian decision theory of an improving situation1
References
Reigner BG, Blesch KS. Estimating the starting dose for entry into humans: principles and practice. Eur J Clin Pharmacol 2002; 57 (12): 835–45
Zhou Y. Choice of designs and doses for early phase trials. Fundam Clin Pharmacol 2004; 18 (3): 373–8
Chevret S. Statistical methods for dose-finding experiments: statistics in practice. Chichester: John Wiley & Sons Ltd, 2006
Rosenberger WF, Haines LM. Competing designs for phase I clinical trials: a review. Stat Med 2002; 21 (18): 2757–70
O’Quigley J, Pepe M, Fisher L. Continual reassessment method: a practical design for phase 1 clinical trials in cancer. Biometrics 1990; 46 (1): 33–48
Whitehead J, Brunier H. Bayesian decision procedures for dose determining experiments. Stat Med 1995; 14 (9–10): 885–93; discussion 895–9
Babb J, Rogatko A, Zacks S. Cancer phase I clinical trials: efficient dose escalation with overdose control. Stat Med 1998; 17 (10): 1103–20
Shih WJ, Lin Y. Traditional and modified algorithm-based designs for phase I cancer clinical trials. In: Chevret S, editor. Statistical methods for dose-finding experiments. Chichester: John Wiley & Sons Ltd, 2006: 61–90
O’Quigley J, Zohar S. Experimental designs for phase I and phase I/II dose-finding studies. Br J Cancer 2006; 94 (5): 609–13
Faries D. Practical modifications of the continual reassessment method for phase I cancer clinical trials. J Biopharm Stat 1994; 4 (2): 147–64
Goodman SN, Zahurak ML, Piantadosi S. Some practical improvements in the continual reassessment method for phase I studies. Stat Med 1995; 14 (11): 1149–61
Korn EL, Midthune D, Chen TT, et al. A comparison of two phase I trial designs. Stat Med 1994; 13 (18): 1799–806
Storer BE. Design and analysis of phase I clinical trials. Biometrics 1989; 45 (3): 925–37
Simon R, Freidlin B, Rubinstein L, et al. Accelerated titration designs for phase I clinical trials in oncology. J Natl Cancer Inst 1997; 89 (15): 1138–47
Dancey J, Freidlin B, Rubinstein LV. Accelerated titration designs. In: Chevret S, editor. Statistical methods for dose-finding experiments. Chichester: John Wiley & Sons Ltd, 2006: 91–113
Rosenberger WF. New directions in adaptive designs. Stat Sci 1996; 11 (2): 137–49
Gasparini M, Eisele J. A curve-free method for phase I clinical trials. Biometrics 2000; 56 (2): 609–15
Rogatko A, Babb JS, Tighiouart M, et al. New paradigm in dose-finding trials: patient-specific dosing and beyond phase I. Clin Cancer Res 2005; 11 (15): 5342–6
Whitehead J, Williamson D. Bayesian decision procedures based on logistic regression models for dose-finding studies. J Biopharm Stat 1998; 8 (3): 445–67
Ivanova A. A new dose-finding design for bivariate outcomes. Biometrics 2003; 59 (4): 1001–7
O’Quigley J, Hughes MD, Fenton T. Dose-finding designs for HIV studies. Biometrics 2001; 57 (4): 1018–29
Thall PF, Cook JD. Dose-finding based on efficacy-toxicity trade-offs. Biometrics 2004; 60 (3): 684–93
Whitehead J, Zhou Y, Stevens J, et al. An evaluation of a bayesian method of dose escalation based on bivariate binary responses. J Biopharm Stat 2004; 14 (4): 969–83
Zohar S, O’Quigley J. Identifying the most successful dose (MSD) in dose-finding studies in cancer. Pharm Stat 2006; 5 (3): 187–99
Zohar S, Chevret S. Recent developments in adaptive designs for phase I/II dose- finding studies. J Biopharm Stat. In press
Levy V, Zohar S, Bardin C, et al. A phase I dose-finding and pharmacokinetic study of subcutaneous semisynthetic homoharringtonine (ssHHT) in patients with advanced acute myeloid leukaemia. Br J Cancer 2006; 95 (3): 253–9
Zohar S, O’Quigley J. Optimal designs for estimating the most successful dose. Stat Med 2006; 25 (24): 4311–20
Rogatko A, Babb JS, Wang H, et al. Patient characteristics compete with dose as predictors of acute treatment toxicity in early phase clinical trials. Clin Cancer Res 2004; 10 (14): 4645–51
International Conference on Harmonisation. E9: statistical principles for clinical trials. 1998 Sep [online]. Available from URL: http://www.fda.gov/cder/gui-dance/ICH_E9-fnl.PDF [Accessed 2007 Oct 30]
Acknowledgements
No sources of funding were used to assist in the preparation of this review. The author has no conflicts of interest that are directly relevant to the content of this review.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zohar, S., Levy, V. Dose Estimation. Pharm Med 22, 35–40 (2008). https://doi.org/10.1007/BF03256680
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF03256680