Abstract
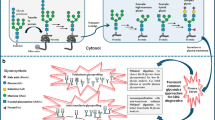
Congenital disorders of glycosylation (CDG) are being recognized as a rapidly growing and complex group of disorders. The pathophysiology results from depressed synthesis or remodeling of oligosaccharide moieties of glycoproteins. The ultimate result is the formation of abnormal glycoproteins affecting their structure and metabolic functions. The most thoroughly studied subset of CDG are the type I defects affecting N-glycosylation. Causal mutations occur in at least 12 different genes which encode primarily monosaccharide transferases necessary for N-glycosylation in the endoplasmic reticulum. The broad clinical presentation of these glycosylation defects challenge clinicians to test for these defects in a variety of clinical settings.
The first described CDG was a phosphomannomutase deficiency (CDG-Ia). The original method used to define the glycosylation defect was isoelectric focusing (IEF) of transferrin. More recently, the use of other charge separation methods and electrospray-mass spectrometry (ESI-MS) has proven valuable in detecting type I CDG defects. By mass resolution, the under-glycosylation of transferrin is characterized as the total absence of one or both N-linked oligosaccharide. Beyond providing a new understanding of the structure of transferrin in type I CDG patients, it is adaptable to high throughput serum analysis.
The use of transferrin under-glycosylation to detect the type I CDG provides limited insight into the specific site of the defect in oligosaccharide assembly since its value is constrained to observation of the final product of glycoprotein synthesis. New analytical targets and tools are converging with the clinical need for diagnosis of CDG. Defining the biosynthetic sites responsible for specific CDG phenotypes is in progress, and ten more type I defects have been putatively identified.
This review discusses current methods, such as IEF and targeted proteomics using mass spectrometry, that are used routinely to test for type I CDG disorders, along with some newer approaches to define the defective synthetic sites responsible for the type I CDG defects. All diagnostic endeavors are followed by the quest for a reliable treatment. The isolated success of CDG-Ib treatment will be described with the hope that this may expand to other type I CDG disorders.







Similar content being viewed by others
References
Marquardt T, Denecke J. Congenital disorders of glycosylation: review of their molecular bases, clinical presentations and specific therapies. Eur J Pediatr 2003 Jun; 162(6): 359–79
Aebi M, Helenius A, Schenk B, et al. Carbohydrate-deficient glycoprotein syndromes become congenital disorders of glycosylation: an updated nomenclature for CDG. First International Workshop on CDGS. Glycoconj J 1999 Nov; 16(11): 669–71
Jaeken JM, Vanderschueren-Lodeweyckx P, Casaer L, et al. Familial psychomotor retardation with markedly fluctuating serum prolactin, FSH and GH levels, partial TBG deficiency increased serum arylsulphatase A and increased CSF protein: a new syndrome? Pediatr Res 1980; 14: 179
Jaeken J, van Eijk HG, van der Heul C, et al. Sialic acid-deficient serum and cerebrospinal fluid transferrin in a newly recognized genetic syndrome. Clin Chim Acta 1984 Dec 29; 144(2–3): 245–7
Babovic-Vuksanovic D, Patterson MC, Schwenk WF, et al. Severe hypoglycemia as a presenting symptom of carbohydrate-deficient glycoprotein syndrome. J Pediatr 1999 Dec; 135 (6): 775–81
Stibler H, Jaeken J. Carbohydrate deficient serum transferrin in a new systemic hereditary syndrome. Arch Dis Child 1990 Jan; 65(1): 107–11
O’Brien JF. Methods for detection of carbohydrate-deficient glycoprotein syndromes. Semin Pediatr Neurol 2005 Sep; 12(3): 159–62
Kranz C, Denecke J, Lehrman MA, et al. A mutation in the human MPDU1 gene causes congenital disorder of glycosylation type If (CDG-If). J Clin Invest 2001 Dec; 108(11): 1613–9
Arndt T. Carbohydrate-deficient transferrin as a marker of chronic alcohol abuse: a critical review of preanalysis, analysis, and interpretation. Clin Chem 2001 Jan; 47(1): 13–27
Bean P, Peter JB. Allelic D variants of transferrin in evaluation of alcohol abuse: differential diagnosis by isoelectric focusing-immunoblotting-laser densitometry. Clin Chem 1994 Nov; 40 (11 Pt 1): 2078–83
Fenn JB, Mann M, Meng CK, et al. Electrospray ionization for mass spectrometry of large biomolecules. Science 1989 Oct 6; 246(4926): 64–71
Wada Y, Nishikawa A, Okamoto N, et al. Structure of serum transferrin in carbohydrate-deficient glycoprotein syndrome. Biochem Biophys Res Commun 1992 Dec 15; 189(2): 832–6
Lacey JM, Bergen HR, Magera MJ, et al. Rapid determination of transferrin isoforms by immunoaffinity liquid chromatography and electrospray mass spectrometry. Clin Chem 2001 Mar; 47(3): 513–8
Bergen 3rd HR, Zeldenrust SR, Butz ML, et al. Identification of transthyretin variants by sequential proteomic and genomic analysis. Clin Chem 2004 Sep; 50(9): 1544–52
Hahn SH, Minnich SJ, O’Brien JF. Stabilization of hypoglycosylation in a patient with congenital disorder of glycosylation type Ia. J Inherit Metab Dis 2006 Feb; 29(1): 235–7
Mills K, Mills P, Jackson M, et al. Diagnosis of congenital disorders of glycosylation type-I using protein chip technology. Proteomics 2006 Apr; 6(7): 2295–304
Jaeken J, Matthijs G, Carchon H, et al. Defects of N-glycan synthesis. In: Scriver CR, Beaudet AL, Sly WS, et al., editors. The metabolic and molecular bases of inherited disease. 8th ed. New York: The McGraw-Hill Companies, 2001: 1601–22
Korner C, Knauer R, Stephani U, et al. Carbohydrate deficient glycoprotein syndrome type IV: deficiency of dolichyl-P-Man:Man(5)GlcNAc(2)-PP-dolichyl mannosyltransferase. Embo J 1999 Dec 1; 18(23): 6816–22
Imbach T, Schenk B, Schollen E, et al. Deficiency of dolichol-phosphate-mannose synthase-1 causes congenital disorder of glycosylation type Ie. J Clin Invest 2000 Jan; 105(2): 233–9
Eklund EA, Newell JW, Sun L, et al. Molecular and clinical description of the first US patients with congenital disorder of glycosylation Ig. Mol Genet Metab 2005 Jan; 84(1): 25–31
Thiel C, Schwarz M, Peng J, et al. A new type of congenital disorders of glycosylation (CDG-Ii) provides new insights into the early steps of dolichol-linked oligosaccharide biosynthesis. J Biol Chem 2003 Jun 20; 278(25): 22498–505
Wu X, Rush JS, Karaoglu D, et al. Deficiency of UDP-GlcNAc:dolichol phosphate N-acetylglucosamine-1 phosphate transferase (DPAGT1) causes a novel congenital disorder of Glycosylation Type Ij. Hum Mutat 2003 Aug; 22(2): 144–50
Schwarz M, Thiel C, Lubbehusen J, et al. Deficiency of GDP-Man:GlcNAc2-PP-dolichol mannosyltransferase causes congenital disorder of glycosylation type Ik. Am J Hum Genet 2004 Mar; 74(3): 472–81
Frank CG, Grubenmann CE, Eyaid W, et al. Identification and functional analysis of a defect in the human ALG9 gene: definition of congenital disorder of glycosylation type IL. Am J Hum Genet 2004 Jul; 75(1): 146–50
Gao N. Fluorophore-assisted carbohydrate electrophoresis: a sensitive and accurate method for the direct analysis of dolichol pyrophosphate-linked oligosaccharides in cell cultures and tissues. Methods 2005 Apr; 35(4): 323–7
Papac DI, Briggs JB, Chin ET, et al. A high-throughput microscale method to release N-linked oligosaccharides from glycoproteins for matrix-assisted laser desorption/ionization time-of-flight mass spectrometric analysis. Glycobiology 1998 May; 8(5): 445–54
Sagi D, Kienz P, Denecke J, et al. Glycoproteomics of N-glycosylation by in-gel deglycosylation and matrix-assisted laser desorption/ionisation-time of flight mass spectrometry mapping: application to congenital disorders of glycosylation. Proteomics 2005 Jul; 5(10): 2689–701
Wada Y. Mass spectrometry for congenital disorders of glycosylation, CDG. J Chromatogr B Analyt Technol Biomed Life Sci 2006 Jun 21; 838(1): 3–8
Helenius A, Aebi M. Roles of N-linked glycans in the endoplasmic reticulum. Annu Rev Biochem 2004; 73: 1019–49
Sturiale L, Barone R, Fiumara A, et al. Hypoglycosylation with increased fucosylation and branching of serum transferrin N-glycans in untreated galactosemia. Glycobiology 2005 Dec; 15(12): 1268–76
Miura Y, Tay SK, Aw MM, et al. Clinical and biochemical characterization of a patient with congenital disorder of glycosylation (CDG) IIx. J Pediatr 2005 Dec; 147(6): 851–3
Allen JP, Anton R. Biomarkers as aids to identification of relapse in alcoholic patients. Recent Dev Alcohol 2003; 16: 25–38
Kawahara H, Matsuda Y, Tsuchishima M, et al. Effects of ethanol and acetaldehyde on the maturation of hepatic secretory glycoproteins. Alcohol Alcohol Suppl 1993; 1A: 29–35
Harasymiw J, Bean P. The combined use of the early detection of alcohol consumption (EDAC) test and carbohydrate-deficient transferrin to identify heavy drinking behaviour in males. Alcohol 2001 Jul–Aug; 36(4): 349–53
Lanz C, Marti U, Thormann W. Capillary zone electrophoresis with a dynamic double coating for analysis of carbohydrate-deficient transferrin in human serum: precision performance and pattern recognition. J Chromatogr A 2003 Sep 26; 1013(1–2): 131–47
Jeppsson JO, Arndt T, Schellenberg F, et al. Toward standardization of carbohydrate-deficient transferrin (CDT) measurements: I. Analyte definition and proposal of a candidate reference method. Clin Chem Lab Med 2007; 45(4): 558–62
Rush JS, Panneerselvam K, Waechter CJ, et al. Mannose supplementation corrects GDP-mannose deficiency in cultured fibroblasts from some patients with congenital disorders of glycosylation (CDG). Glycobiology 2000 Aug; 10(8): 829–35
Niehues R, Hasilik M, Alton G, et al. Carbohydrate-deficient glycoprotein syndrome type Ib: phosphomannose isomerase deficiency and mannose therapy. J Clin Invest 1998 Apr 1; 101(7): 1414–20
Westphal V, Srikrishna G, Freeze HH. Congenital disorders of glycosylation: have you encountered them? Genet Med 2000 Nov–Dec; 2(6): 329–37
Acknowledgments
No sources of funding were used to assist in the preparation of this review. The authors have no conflicts of interest that are directly relevant to the content of this review.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Babovic-Vuksanovic, D., O’Brien, J.F. Laboratory Diagnosis of Congenital Disorders of Glycosylation Type I by Analysis of Transferrin Glycoforms. Mol Diag Ther 11, 303–311 (2007). https://doi.org/10.1007/BF03256251
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF03256251