Abstract
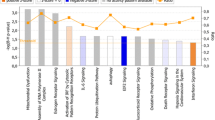
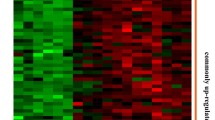
The lipoteichoic acid (LTA) of Staphylococcus aureus (aLTA) and Lactobacillus plantarum (pLTA) engage the same toll-like receptor 2 (TLR2) signaling pathway but exert different effects on innate immunity and inflammation. The mechanisms underlying these differential effects are not yet clear. Human oligonucleotide microarrays were used to investigate the transcriptome of human THP-1 monocytes upon exposure to aLTA or pLTA, and differential gene expression profiles were observed between the aLTA- and pLTA-treated cells. The expression level of 1,302 genes in aLTAtreated cells increased more than 2-fold; some of which have been implicated in immune or inflammatory responses, cell adhesion, cell signal transduction, transcription factors, anion transport, proteolysis, and oxidative processes. Particularly, a variety of genes that encode cytokines and chemokines, and TLR signaling-related molecules belonging to the tumor necrosis factor receptor-associated factor (TRAF), nuclear factor-kappa B, and signal transducer and activator of transcription families were remarkably up-regulated by aLTA stimulation. In contrast, pLTA treatment altered the expression of only 90 genes by more than 1.5-fold, and these genes were not correlated with innate immunity, inflammation or other related processes. The different effects mediated by aLTA and pLTA were further verified and compared by analysis of the expression of a selected group of genes, including TRAFs and some cytokines and chemokines, using real time-polymerase chain reaction and ELISA. These data suggest that aLTA and pLTA have different immunomodulatory potentials. Compared with pLTA, aLTA is a stronger stimulator and impacts the expression of many innate immunity- and/or inflammation-related genes.
Similar content being viewed by others
References
Baeuerle PA and Baltimore D (1998) I kappa B: A specific inhibitor of the NF-kappa B transcription factor. Science 242, 540–546.
Behr T, Fischer W, Peter-Katalinic J, and Egge H (1992) The structure of pneumococcal lipoteichoic acid: Improved preparation, chemical and mass spectrometric studies. Eur J Biochem 207, 1063–1075.
Beutler B (2004) Inferences, questions and possibilities in Toll-like receptor signaling. Nature 430, 257–283.
Bhakdi S, Klonisch T, Nuber P, and Fischer W (1991) Stimulation of monokine production by lipoteichoic acids. Infect Immunol 59, 4614–4620.
Bloksma N, de Heer E, van Dijk H, and Willers JM (1979) Adjuvanticity of lactobacilli. I. Differential effects of viable and killed bacteria. Clin Exp Immunol 37, 367–375.
Bradley JR and Pober JS (2001) Tumor necrosis factor receptor-associated factors (TRAFs). Oncogene 20, 6482–6491.
Chung JY, Park YC, Ye H, and Wu H (2002) All TRAFs are not created equal: Common and distinct molecular mechanisms of TRAF-mediated signal transduction. J Cell Sci 115, 679–688.
Deininger S, Stadelmaier A, von Aulock S, Morath S, Schmidt RR, and Hartung T (2003) Definition of structural prerequisites for lipoteichoic acid-inducible cytokine induction by synthetic derivatives. J Immunol 170, 4134–4138.
Ellingsen E, Morath S, Flo T, Schromm A, Hartung T, Thiemermann C, Espevik T, Golenbock D, Foster D, Solberg R, Aasen A, and Wang J (2002) Induction of cytokine production in human T cells and monocytes by highly purified lipoteichoic acid: Involvement of Toll-like receptors and CD14. Med Sci Monit 8, 149–156.
Fischer W, Mannsfeld T, and Hagen G (1990) On the basic structure of poly (glycerophosphate) lipoteichoic acids. Biochem Cell Biol 68, 33–43.
Fujioka S, Niu JG, Schmidt C, Sclabas GM, Peng BL, Uwagawa T, Li ZK, Evans DB, Abbruzzese JL, and Chiao PJ, (2004) NF-κB and AP-1 connection: Mechanism of NF-κB-dependent regulation of AP-1 activity. Mol Cell Biol 24, 7806–7819.
Georgieva RN, IIiev IN, Chipeva VA, Dimitonova SP, Samelis J, and Danova ST (2008) Identification and in vitro characterization of Lactobacillus plantarum strains from artisanal Bulgarian white brined cheeses. J Basic Microbiol 48, 234–244.
Ginsburg I (2002) Role of lipoteichoic acid in infection and inflammation. Lancet Infect Dis 2, 171–179.
Grangette C, Nutten S, Palumbo E, Morath S, Hermann C, Dewulf J, Pot B, Hartung T, Hols P, and Mercenier A (2005) Enhanced antiinflammatory capacity of Lactobacillus plantarum mutant synthesizing modified teichoic acids. Proc Natl Acad Sci USA 102, 10321–10326.
Greengerg JW, Fischer W, and Joiner KA (1996) Influence of lipoteichoic acid structure on recognition by the macrophage scavenger receptor. Infect Immunol 64, 3318–3325.
Guo Y, Guo H, Zhang L, Xie H, Zhao X, Wang F, Li Z, Wang Y, Ma S, Tao J, Wang W, Zhou Y, Yang W, and Cheng J (2005) Genomic analysis of anti-hepatitis B virus (HBV) activity by small interfering RNA and lamivudine in stable HBV-producing cells. J Virol 79, 14392–14403.
Ha CG, Cho JK, Lee CH, Chai YG, Ha YA, and Shin SH (2006) Cholesterol lowering effect of Lactobacillus plantarum isolated from human feces. J Microbiol Biotechnol 16, 1201–1209.
Han SH, Kim JK, Martin M, Michalek SM, and Nahm MH (2003) Pneumococcal lipoteichoic acid (LTA) is not as potent as Staphylococcal LTA in stimulating Toll-like receptor 2. Infect Immunol 71, 5541–5548.
Hayden MS and Ghosh S (2008) Shared principles in NF-κB signaling. Cell 132, 344–362.
Jang HS, Chung HS, Ko EJ, Shin JS, Shin MK, Hong MC, Kim YS, Min BI, and Bae HS (2009) Microarray analysis of gene expression profiles in response to treatment with bee venom in lipopolysaccharide activated RAW 264.7 cells. J Ethnopharmacol 121, 213–220.
Kim HG, Gim MG, Kim JY, Hwang HJ, Ham MS, Lee JM, Hartung T, Park JW, Han SH, and Chung DK (2007) Lipoteichoic acid from Lactobacillus plantarum elicits both the production of Interleukin-23p19 and suppression of pathogen-mediated Interleukin-10 in THP-1 cells. FEMS Immunol Med Microbiol 49, 205–214.
Kim HG, Kim NR, Gim MG, Lee JM, Lee SY, Ko MY, Kim JY, Han SH, and Chung DK (2008a) Lipoteichoic acid isolated from Lactobacillus plantarum inhibits lipopolysaccharide-induced TNF-α production in THP-1 cells and endotoxin shock in mice. J Immunol 180, 2553–2561.
Kim HG, Lee SY, Kim NR, Ko MY, Lee JM, Yi TH, Chung SK, and Chung DK (2008b) Inhibitory effects of Lactobacillus plantarum lipoteichoic acid (LTA) on Staphylococcus aureus LTA-induced tumor necrosis factor-alpha production. J Microbiol Biotechnol 18, 1191–1196.
Lee HM and Lee YH (2006) Isolation of Lactobacillus plantarum from kimchi and its inhibitory activity on the adherence and growth of Helicobacter pylori. J Microbiol Biotechnol 16, 1513–1517.
Malcolm KC, Arndt PG, Manos EJ, Jones DA, and Worthen GS (2003) Microarray analysis of lipopolysaccharide-treated human neutrophils. Am J Physiol Lung Cell Mol Physiol 284, 663–670.
Matsusaka T, Fujikawa K, Nishio Y, Mukaida N, Matsushima K, Kishimoto T, and Akira S (1993) Transcription factors NF-IL6 and NF-kappa B synergistically activate transcription of the inflammatory cytokines, interleukin 6 and interleukin 8. Proc Natl Acad Sci USA 90, 10193–10197.
Mattsson E, Verhage L, Rollof J, Fleer A, Verhoef J, and van Dijk H (1993) Peptidoglycan and teichoic acid from Staphylococcus epidermidis stimulate human monocytes to release tumour necrosis factor-alpha, interleukin-1β and interleukin-6. FEMS Immunol Med Microbiol 7, 281–287.
Miggin SM and O’Neill LAJ (2006) New insights into the regulation of TLR signaling. J Leukoc Biol 80, 1–7.
Mohamadzadeh M, Olson S, Kalina WV, Ruthel G, Demmin GL, Warfield KL, Bavari S, and Klaenhammer TR (2005) Lactobacilli activate human dendritic cells that skew T cells toward T helper 1 polarization. Proc Natl Acad Sci USA 102, 2880–2885.
Oyoshi MK, Bryce P, Goya S, Pichavant M, Umetsu DT, Oettgen HC, and Tsitsikov EN (2008) TNF receptor-associated factor 1 expressed in resident lung cells is required for the development of allergic lung inflammation. J Immunol 180, 1878–1885.
Patterson TA, Lobenhofer EK, Fulmer-Smentek SB, Collins PJ, Chu TM, Bao W, Fang H, Kawasaki ES, Hager J, Tikhonova IR, Walker SJ, Zhang L, Hurban P, de Longueville F, Fuscoe JC, Tong W, Shi L, and Wolfinger RD (2006) Performance comparison of one-color and two-color platforms within the MicroArray Quality Control (MAQC) project. Nat Biotechnol 24, 1140–1150.
Schroder NWJ, Morath S, Alexander C, Hamann L, Hartung T, Zahringer U, Gobel UB, Weber JR, and Schumann RR (2003) Lipoteichoic acid (LTA) of Streptococcus Pneumonia and Staphylococcus aureus activates immune cells via Toll-like receptor (TLR)-2, lipopolysaccharide-binding protein (LBP), and CD14, whereas TLR-4 and MD-2 are not involved. J Biol Chem 278, 15587–15594.
Seo HS, Michalek SM, and Nahm MH (2008) Lipoteichoic acid is important in innate immune responses to Gram-positive bacteria. Infect Immunol 76, 206–213.
Standiford TJ, Arenberg DA, Danforth JM, Kunkel SL, Van Otteren GM, and Strieter RM (1994) Lipoteichoic acid induces secretion of interleukin-8 from human blood monocytes: A cellular and molecular analysis. Infect Immunol 62, 119–125.
Takeuchi O, Hoshino K, and Akira S (2000) Cutting edge: TLR2-deficient and MyD88-deficient mice are highly susceptible to Staphylococcus aureus infection. J Immunol 165, 5392–5396.
Velez MP, Verhoeven TLA, Draing C, Aulock SV, Pfitzenmaier M, Geyer A, Lambrichts I, Grangette C, Pot B, Vanderleyden J, and De Keersmaecker SCJ (2007) Functional analysis of D-alanylation of lipoteichoic acid in the probiotic strain Lactobacillus rhamnosus GG. Appl Environ Microbiol 73, 3595–3604.
Yang YH, Dudoit S, Luu P, Lin DM, Peng V, Ngai J, and Speed TP (2002) Normalization for cDNA microarray data: a robust composite method addressing single and multiple slide systematic variation. Nucleic Acids Res 30, e15.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zeng, RZ., Kim, H.G., Kim, N.R. et al. Differential gene expression profiles in human THP-1 monocytes treated with Lactobacillus plantarum or Staphylococcus aureus lipoteichoic acid. J Korean Soc Appl Biol Chem 54, 763–770 (2011). https://doi.org/10.1007/BF03253157
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF03253157