Abstract
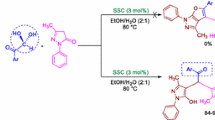
In this work, a green, simple and highly efficient procedure for the synthesis of bis(indolyl)methanes and 4,4′- (arylmethylene)-bis(3-methyl-1-phenyl-1H-pyrazol-5-ol)s [as an important class of bis(pyrazolyl)methanes] is described. The condensation of indoles or 1-phenyl-3-methylpyrazol-5-one with carbonyl compounds catalyzed by poly(ethylene glycol)-bound sulfonic acid (PEG-SO3H) in water affords the title compounds in high yields and relatively short reaction times.
Similar content being viewed by others
References
A. Curzons, C.C. Constable, V.L. Cunningham, Clean Prod. Process. 1 (1999)
R. Gani, C. Jiménez-Gonzalez, A. Kate, P.A. Crafts, M. Jones, L. Powell, J.H. Atherton, J.L. Cordiner, Chem. Eng. 1 (2006) 30
A. Zare, A. Hasaninejad, A. Khalafi-Nezhad, A.R. Moosavi-Zare, M.H. Beyzavi, F. Khedri, F. Asadi, N. Hayati, A. Asifi, J. Iran. Chem. Soc. 7 (2010) 461
A. Shaabani, R. Ghadari, A. Rahmati, A.H. Rezayan, J. Iran. Chem. Soc. 6 (2009) 710
R.S. Bhosale, S.R. Sarda, R.P. Giram, D.S. Raut, S.P. Parwe, S.S. Ardhapure, R.P. Pawar, J. Iran. Chem. Soc. 6 (2009) 519.
C.-J. Li, T.-H. Chan, Organic Reactions in Aqueous Media, John Wiley & Sons, New York, 1997.
Organic Synthesis in Water, P.A. Grieco (Ed.), Blackie Academic and Professional, London, 1998
U.M. Lindstrom, Chem. Rev. 102 (2002) 2751
S. Kobayashi, K. Manabe, Acc. Chem. Res. 35 (2002) 209
S. Narayan, J. Muldoon, M.G. Fin, V.V. Folkin, H.C. Kolb, K.B. Sharpless, Angew. Chem., Int. Ed. 44 (2005) 3275
C.-J. Li, Chem. Rev. 93 (1993) 2023
N. Azizi, E. Akbari, M.R. Saidi, J. Iran. Chem. Soc. 6 (2009) 165
N. Azizi, A. Khajeh Amiri, M. Bolourtchian, M.R. Saidi, J. Iran. Chem. Soc. 6 (2009) 749
M.M. Heravi, A. Ghods, F. Derikvanda, K. Bakhtiari, F.F. Bamoharram, J. Iran. Chem. Soc. 6 (2009) 615
H. Mehrabi, H. Abusaidi, J. Iran. Chem. Soc. 6 (2009) 890
A. Hasaninejad, A. Zare, M.R. Mohammadizadeh, Z. Karami, J. Iran. Chem. Soc. 6 (2009) 153.
S. Ribe, P. Wipf, Chem. Commun. (2001) 299.
S.-J. Tu, J.-F. Zhou, Z.-S. Lu, X. Deng, D.-Q. Shi, S.-H. Wang, Synth. Commun. 32 (2002) 3063.
J. Eames, M. Watkinson, J. Org. Chem. (2001) 1213.
C.C. Tzschucke, C. Markert, W. Bannwarth, S. Roller, A. Hebel, R. Haag, Angew. Chem. 114 (2002) 4136.
L. Wang, J. Han, H. Tian, J. Sheng, Z. Fan, X. Tang, Synlett (2005) 37.
X. Wang, Z. Quan, F. Wang, M. Wang, Z. Zhang, Z. Li, Synth. Commun. 36 (2006) 451.
A.R. Kiasat, M.F. Mehrjardi, Catal. Commun. 9 (2008) 1497.
J.K. Porter, C.W. Bacon, J.D. Robbins, D.S. Himmelsbach, H.C. Higman, J. Agric. Food Chem. 25 (1977) 88
T. Osawa, M. Namiki, Tetrahedron Lett. 24 (1983) 4719
G. Bifulco, I. Bruno, R. Riccio, J. Lavayre, G. Bourdy, J. Nat. Prod. 57 (1994) 1254
R. Bell, S. Carmeli, N. Sar, J. Nat. Prod. 57 (1994) 1587
T.R. Garbe, M. Kobayashi, N. Shimizu, N. Takesue, M. Ozawa, H. Yukawa, J. Nat. Prod. 63 (2000) 596
M.A. Zeligs, J. Med. Food, 1 (1998) 67
C. Grubbs, V. Steele, T. Casebolt, M.M. Juliana, I. Eto, L.M. Whitaker, Anticancer Res. 15 (1995) 709
S.H. Benabadji, R. Wen, J. Zheng, X. Dong, S. Yuan, Acta Pharmacol. Sin. 25 (2004) 666
M.J. Anderton, M.M. Manson, R. Verschoyle, A. Gescher, W.P. Steward, M.L. Williams, D.E. Mager, Drug Metab. Dispos. 32 (2004) 632.
M. Shiri, M.A. Zolfigol, H.G. Kruger, Z. Tanbakouchian, Chem. Rev. 110 (2010) 2250
G.W. Gribble, J. Chem. Soc., Perkin Trans. 1 (2000) 1045
R.J. Sundberg, The Chemistry of Indoles, Academic Press, New York, 1970
R.J. Sundberg, Indoles, Academic Press, San Diego, CA, 1996
J.A. Joule, Science of Synthesis (indoles), E.J. Thomas (Ed.), Georg Thieme Verlag, Stuttgart, New York, 2000, Vol. 10, pp. 361–652.
M. Chakrabarty, R. Basak, Y. Harigaya, Heterocycles 55 (2001) 2431.
B.P. Bandgar, K.A. Shaikh, Tetrahedron Lett. 44 (2003) 1959.
H. Koshima, W. Matsuaka, J. Heterocycl. Chem. 39 (2002) 1089.
A. Hasaninejad, A. Parhami, A. Zare, A. Khalafi- Nezhad, A. Nasrolahi Shirazi, A.R. Moosavi Zare, Polish J. Chem. 82 (2008) 565.
A. Hasaninejad, A. Zare, H. Sharghi, M. Shekouhy, R. Khalifeh, A. Salimi Beni, A.R. Moosavi Zare, Can. J. Chem. 85 (2007) 416.
A. Hasaninejad, A. Zare, H. Sharghi, K. Niknam, M. Shekouhy, ARKIVOC xiv (2007) 39.
A. Khalafi-Nezhad, A. Parhami, A. Zare, A.R. Moosavi Zare, A. Hasaninejad, F. Panahi, Synthesis (2008) 617.
A. Zare, A. Parhami, A.R. Moosavi-Zare, A. Hasaninejad, A. Khalafi-Nezhad, M.H. Beyzavi, Can. J. Chem. 87 (2009) 416
J.S. Yadav, B.V.S. Reddy, S. Sunitha, Adv. Synth. Catal. 45 (2003) 349
D.G. Gu, S.J. Ji, Z.Q. Jiang, M.F. Zhou, T.P. Loh, Synlett (2005) 959.
J.S. Yadav, B.V.S. Reddy, C.V.S.R. Murthy, G.M. Kumar, C. Madan, Synthesis (2001) 783
H. Firouzabadi, N. Iranpour, M. Jafarpour, A. Ghaderi, J. Mol. Cat. A: Chem. 253 (2006) 249
J.S. Yadav, B.V.S. Reddy, G. Satheesh, A. Prabhakar, A.C. Kunwar, Tetrahedron Lett. 44 (2003) 2221
G. Babu, N. Sridhar, P.T. Perumal, Synth. Commun. 30 (2000) 1609
D.P. Chen, L.B. Yu, P.G. Wang, Tetrahedron Lett. 37 (1996) 4467
R. Nagarajan, P.T. Perumal, Tetrahedron 58 (2000) 1229
X.L. Mi, S.Z. Luo, J.Q. He, J.P. Chen, Tetrahedron Lett. 5 (2004) 4567
L. Wang, J. Han, H. Tian, J. Sheng, Z. Fan, X. Tang, Synlett (2005) 37.
S. Kobayashi, M. Araki, M. Yasuda, Tetrahedron Lett. 6 (1995) 5773.
J. Elguero, A.R. Katritzky, C.W. Rees (Eds.), Comprehensive Heterocyclic Chemistry: Pyrazoles and their Benzo Derivatives, Vol. 5, Pergamon Press, Oxford, 1984
H. Nakagawa, R. Ohyama, A. Kimata, T. Suzuki, N. Miyata, Bioorg. Med. Chem. Lett. 16 (2006) 5939
C. Coimra, F. Boris-Möller, M. Drqke, T. Wieloch, Acta Neuropathology 92 (1996) 447.
T. Kessler, T. Aybek, G. Neidhart, S. Dogan, D. Bremerich, D. Lischke, C. Byhahan, J. Cardiothoracic and Vascular Anesthesia 19 (2005) 32.
S. Sugiura, S. Ohno, O. Ohtani, K. Izumi, T. Kitamikado, H. Asai, K. Kato, J. Med. Chem. 20 (1977) 80.
L.C. Behr, R. Fusco C.H., Jarboe, The Chemistry of Heterocyclic Compounds, Pyrazoles, Pyrazolines, Pyrazolidines, Indazoles and Condensed Rings, A. Weissberger (Ed.), Interscience Publishers, New York, 1967.
C.E. Rosiere, M.I. Grossman, Science 113 (1951) 651.
D.M. Bailey, P.E. Hansen, A.G. Hlavac, E.R. Baizman, J. Pearl, A.F. Defelice, M.E. Feigenson, J. Med. Chem. 28 (1985) 256.
R.N. Mahajan, F.H. Havaldar, P.S. Fernandes, J. Ind. Chem. Soc. 68 (1991) 245.
P.M.S. Chauhan, S. Singh, R.K. Chatterjee, Ind. J. Chem., Sect. B: Org. Chem. Incl. Med. Chem. 32 (1993) 858.
D. Singh, D. Singh, J. Ind. Chem. Soc. 68 (1991) 165.
M. Londershausen, Pestic. Sci. 48 (1996) 269.
The Chemistry of Synthetic Dyes and Pigments, H.A. Lubs (Ed.), American Chemical Society, Washington D.C., 1970.
A.B. Uzoukwu, Polyhedron 12 (1993) 2719
R.C. Maurya, R. Verma, Ind. J. Chem., Sect. A 36 (1997) 596
A.D. Garnovskii, A.I. Uraev, V.I. Minkin, ARKIVOC iii (2004) 29.
D. Singh, D. Singh, J. Chem. Eng. Data 29 (1984) 355.
B.I. Buzykin, T.I. Lonshchakova, Bull. Acad. Sci. USSR, Div. Chem. Sci. (Engl. Transl.) (1971) 2224.
W. Wang, S.-X. Wang, X.-Y. Qin, J.-T. Li, Synth. Commun. 35 (2005) 1263.
M.N. Elinson, A.S. Dorofeev, R.F. Nasybullin, G.I. Nikishin, Synthesis (2008) 1933.
X. Zeng, S. Ji, S. Wang, Tetrahedron 61 (2005) 10235.
M.L. Deb, P.J. Bhuyan, Tetrahedron Lett. 47 (2006) 1441.
J.-B. Meng, D.-M. Du, W.-G. Wang, Y.-M. Wang, Chem. J. Chin. Univ. 15 (1994) 528.
M.A. Zolfigol, P. Salehi, M. Shiri, Z. Tanbakouchian, Catal. Commun. 8 (2007) 173.
K. Tabatabaeian, M. Mamaghani, N. Mahmoodi, A. Khorshidi, Can. J. Chem. 84 (2006) 1541.
K. Sujatha, G. Shanthi, N.P. Selvam, S. Manoharan, P.T. Perumal, M. Rajendran, Bioorg. Med. Chem. Lett. 19 (2009) 4501.
M. Shiri, M.A. Zolfigol, Tetrahedron 65 (2009) 587.
K. Niknam, M.A. Zolfigol, T. Sadabadi, A. Nejati, J. Iran. Chem. Soc. 3 (2006) 318
R. Nagarajan, P.T. Perumal, Synth. Commun. 32 (2002) 105
C.J. Magesh, R. Nagarajan, M. Karthik, P.T. Perumal, Appli. Catal. A: Gen. 266 (2004) 1
L. Wang, J.H. Han, T. Sheng, J.Z. Fan, X. Tang, Synlett (2005) 337
H. Firouzabadi, N. Iranpoor, A.A. Jafari, J. Mol. Catal. A: Chem. 244 (2006) 168
J.S. Yadav, B.V.S. Reddy, P. Sreedhar, R.S. Rao, K. Nagaiah, Synthesis (2004) 2381
A. Shaabani, E. Soleimani, Z. Badri, Synth. Commun. 37 (2007) 629
B. Staskun, J. Org. Chem. 29 (1964) 1153.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Hasaninejad, A., Shekouhy, M., Zare, A. et al. PEG-SO3H as a new, highly efficient and homogeneous polymeric catalyst for the synthesis of bis(indolyl)methanes and 4,4′-(arylmethylene)-bis(3-methyl-1-phenyl-1Hpyrazol-5-ol)s in water. JICS 8, 411–423 (2011). https://doi.org/10.1007/BF03249075
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF03249075