Abstract
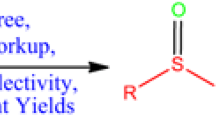
Various types of aromatic and aliphatic sulfides are selectively oxidized to the corresponding sulfoxides in good to excellent yields using 30% H2O2 in the presence of catalytic amounts of N-bromosuccinimide (NBS) in buffered aqueous acetonitrile solution (pH =7.00). The results showed that acid sensitive functional groups such as double bonds, and O,O-acetals remained intact under the described reaction conditions
Similar content being viewed by others
References
G. Soladie, Synthesis (1981) 185.
M.C. Carreno, Chem. Rev. 95 (1995) 1717.
S.G. Davies, T. Loveridge and J.M. Clough, Synlett (1997) 66.
N. Matzaki, A. Sakamoto, N. Nagahashi, M. Soejima, Y.X. Li, T. Imamura, N. Kojima, H. Ohishi, K.I. Sakaquchi, C. Iwata and T. Tanaka, J. Org. Chem. 65 (2000) 3284.
J. Fuhrhop, G. Penzlin, Organic Synthesis, Conceptd, Methods, Stating materials, 2nd ed.; VCH: Weinheim, (1994).
E. Block, Reaction of Organosulfur Compounds; Academic Press: New York, (1978).
M. Madesclaire, Tetrahedron 42 (1986) 5459.
E.G. Mata, Phosphorus Sulfur Silicon Relat. Elem. 117 (1996) 231.
A.Y. Koposov, V.V. Zhdankin, Synthesis (2005) 22.
W. Qian, L. Pei, Synlett (2006) 709.
P. Kelly, S.E. Lawrence and A.R. Maguire, Synlett (2007) s 1501 and the references cited therein.
A. Mckillop, J.A. Tarbin, Tetrahedron Lett. 24 (1983) 1505.
S.W. Kaldor, M. Hammond, Tetrahedron Lett. 32 (1991) 5043.
G.W. Gokel, H.M. Gerdes and D.M. Dishong, J. Org. Chem. 45 (1980) 3634.
R.S. Varma, R.K. Saini and H.M. Meshram, Tetrahedron Lett. 38 (1997) 6525.
H. Firouzabadi, M. Abbassi and B. Karimi, Synth. Commun. 29 (1999) 2527.
T.L Ho, C.M. Wong, Synthesis (1972) 561.
J.H. Clark, Green Chem. 1 (1999) 1.
M.V. Gomez, R. Caballero, E. Vazquez, A. Moreno, A.D.L. Hoz and A. Diaz Ortiz, Green Chem. 9 (2007) 331.
K. Sato, M. Huodo, M. Aoki, X.-Q Zhang and R. Noyori, Tetrahedron 57 (2001) 2469.
P. Lupattelli, R. Ruzziconi, P. Scafato, A. Degl’Innocenti and A.B. Paolobelli, Synth. Commun. 27 (1997) 441.
B. Karimi, M. Ghoreishi-Nezhad and J.H. Clark, Org. Lett. 7 (2005) 625.
M. Matteucci, G. Bhalay and M. Bradly, Org. Lett. 5 (2003) 235.
W.L. Xu, Y.Z. Li, Q.S. Zhang and H.S. Zhu, Synthesis (2004) 227.
G.P. Romanelli, P.G. Vazquez and P. Tundo, Synlett (2005) 75.
T. Yamaguchi, K. Matsumoto, B. Saito and T. Katsuki, Angew. Chem. Int. Ed. 46 (2007) 4729.
M. Mba, I.J. Prins & G. Licini, Org. Lett. 9 (2007) 21.
V. Khanna, G.C. Maikap and J. Iqbal, Tetrahedron Lett. 37 (1996) 3367.
M.M. Dell’ Anna, P. Mastrorilli and C.F. Nobile, J. Mol. Catal. A: Chemical. 108 (1996) 57.
T. Iwahama, S. Sakaguchi and Y. Ishii, Tetrahedron Lett. 39 (1998) 9059.
S.E Martin, L.I. Rossi, Tetrahedron Lett. 42 (2001) 7147.
A. Bravo, B. Dordi, F. Fontana and F. Minisci, J. Org. Chem. 66 (2001) 3232.
Q. De Lucchi, U. Miotti and G. Modena, Org. React. 40 (1991) 157.
B. Karimi, B. Golshani, J. Org. Chem. 65 (2000) 7228.
B. Karimi, G.R. Ebrahimian and H. Seradj, Org. Lett. 1 (1999) 1737.
B. Karimi, H. Seradj, Synlett (2000) 623.
B. Karimi, H. Seradj, Synlett (2001) 519.
B. Karimi, A. Miri Ashtiani, Chem. Lett. (1999) 1199.
B. Karimi, H. Seradj and G.R Ebrahimian, Synlett (1999) 1456.
H. Firouzabadi, N. Iranpoor and B. Karimi, Synthesis (1999) 58.
B. Karimi, H. Seradj and M.R. Tabaei, Synlett (2000) 1798.
B. Karimi, B. Golshani, Synthesis (2002) 784.
B. Karimi, D. Zareyee, Synlett (2002) 346.
H. Firouzabadi, B. Karimi and S. Eslami, Tetrahedron Lett. 40 (1999) 4055.
B. Karimi, J. Maleki, J. Org. Chem. 68 (2003) 4951.
B. Karimi, J. Maleki, Tetrahedron Lett. 43 (2002) 5353.
B. Karmi, H. Hazarkhani and J. Maleki, Synthesis (2005) 279.
Author information
Authors and Affiliations
Additional information
This paper is dedicated to Professor Habib Firouzabadi on the occasion of his 65th birthday and also his retirement
Rights and permissions
About this article
Cite this article
Karimi, B., Zareyee, D. Selective, metal-free oxidation of sulfides to sulfoxides Using 30% hydrogen peroxide catalyzed with N-bromosuccinimide (NBS) under neutral buffered reaction conditions. JICS 5 (Suppl 1), S103–S107 (2008). https://doi.org/10.1007/BF03246497
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF03246497