Abstract
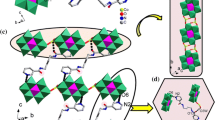
Two novel compounds with formulae [Sn2(pydcH)2(H2O)2O]n, 1, and (4,4′-bpyH2)0.5[Pb(pydc)2(4,4′-bpyH)].4,4′-bpy.4H2O, 2, were obtained from a one-pot reaction between pyridine-2,6-dicarboxylic acid (pydcH2) and 4,4′-bipyridine (4,4′-bpy) with corresponding Sn(II) and Pb(II) salts. In compound 1 with a polymeric structure, each Sn(II) atom is six-coordinated by one water molecule, two (pydcH)− groups and one oxide group resulted in a coordination polymer. Compound 2 has a seven-coordinated environment around Pb(II) atom by two (pydc)2− groups and one (4,4′-bpyH). The anionic complex is balanced by half a (4,4′- bpyH2)2+ as counter ion. There are four uncoordinated water molecules and one 4,4′-bpy in the crystal lattice. Therefore, in compound 2, we have neutral, mono- and biprotonated forms of 4,4′-bipyridine, simultaneously. Several interactions including O-H⋅⋅⋅ O, O-H⋅⋅⋅EN and C-H⋅⋅⋅O hydrogen bonds, ion pairing, C-O⋅⋅⋅π (O⋅⋅⋅Cg 3.324(3) Å and 3.381(3) Å in 1 and O⋅⋅⋅Cg 3.346(4) Å in 2), C-H⋅⋅⋅π (C⋅⋅⋅Cg 3.618(4) Å in 2), and π⋅⋅⋅π stackings (with Cg ⋅⋅⋅ Cg distances of 3.613(2) and 3.641 (2) Å in 2) are present to expand and stabilize the structure. The complexation reactions of bpy and pydc-bpy with Sn2+ and Pb2+ ions in aqueous solution were investigated by potentiometric pH titrations, and the resulting equilibrium constants and species distributions at various pHs for major formed complexes are described.
Similar content being viewed by others
References
Z.-B. Han, Z.-N. Lou, Y.-C. Jiang, L.-T. Zhang, Trans. Met. Chem. 32 (2007) 219.
H. Yin, S.-X. Liu, J. Mol. Struct. 918 (2009) 165.
H. Aghabozorg, F. Manteghi, S. Sheshmani, J. Iran. Chem. Soc. 5 (2008) 184 and references [31–140] therein.
A. Moghimi, S.M. Moosavi, D. Kordestani, B. Maddah, M. Shamsipur, H. Aghabozorg, F. Ramezanipour, G. Kickelbick, J. Mol. Struct. 828 (2007) 38.
a) M.A. Sharif, H. Aghabozorg, A. Shokrollahi, G. Kickelbick, A. Moghimi, M. Shamsipur, Polish J. Chem. 80 (2006) 847; b) A. Moghimi, M.A. Sharif, A. Shokrollahi, M. Shamsipur, H. Aghabozorg, Z. Anorg. Allg. Chem. 631 (2005) 902; c) H. Aghabozorg, A.A. Saei, F. Ramezanipour, Acta Crystallogr. E61 (2005) o3242; d) H. Aghabozorg, F. Manteghi, M. Ghadermazi, Acta Crystallogr. E63 (2007) o4454; e) H. Aghabozorg, M. Ghadermazi, F. Ramezanipour, Acta Crystallogr. E62 (2006) o1143; f) A. Moghimi, S. Sheshmani, A. Shokrollahi, M. Shamsipur, G. Kickelbick, H. Aghabozorg, Z. Anorg. Allg. Chem. 631 (2005) 160; g) H. Aghabozorg, J. Soleimannejad, M.A. Sharif, S. Sheshmani, A. Moghimi, Anal. Sci. 21 (2005) x73.
a) S. Sheshmani, H. Aghabozorg, M. Ghadermazi, Acta Crystallogr. E63 (2007) o2869; b) H. Aghabozorg, M. Ghadermazi, S. Sheshmani, Acta Crystallogr. E62 (2006) o3287; c) H. Aghabozorg, F. Manteghi, M. Ghadermazi, Acta Crystallogr. E64 (2008) o230; d) H. Aghabozorg, S. Daneshvar, E. Motyeian, F. Manteghi, R. Khadivi, M. Ghadermazi, A. Shokrollahi, M. Ghaedi, S. Derki, M. Shamsipur, J. Iran Chem. Soc. 6 (2009) 620; e) H. Aghabozorg, F. Manteghi, M. Ghadermazi, Acta Crystallogr. E64 (2008) o740.
A. Moghimi, R. Alizadeh, A. Shokrollahi, H. Aghabozorg, M. Shamsipur, A. Shockravi, Inorg. Chem. 42 (2003) 1616.
M. Ranjbar, H. Aghabozorg, A. Moghimi, Anal. Sci. 19 (2003) x71.
M. Ranjbar, A. Moghimi, H. Aghabozorg, Anal. Sci. 19 (2003) 803.
Bruker (2007). SMART. Bruker AXS Inc., Madison, Wisconsin, USA.
G.M. Sheldrick, SADABS, v. 2.03, Bruker/Siemens Area Detector Absorption Correction Program, Bruker AXS, Madison, Wisconsin, USA, 2003.
G.M. Sheldrick, SHELXTL, v. 6.12, Structure Determination Software Suite, Bruker AXS, Madison, Wisconsin, USA, 2001.
A.E. Martell, R.J. Motekaitis, Determination and Use of Stability Constants, 2nd ed., VCH, New York, 1992.
G. Schwarzenbach, H. Flaschka, Complexometric Titrations, Methuen, London, 1969.
J. Soleimannejad, H. Aghabozorg, Y. Mohammadzadeh Azar Golenji, J. Attar Gharamaleki, H. Adams, Acta Crystallogr. E64 (2008) m387.
J. Soleimannejad, H. Aghabozorg, S. Hooshmand, Acta Crystallogr. E64 (2008) m564.
J. Soleimannejad, H. Aghabozorg, S. Hooshmand, H. Adams, Acta Crystallogr. E63 (2007) m3089.
J. Soleimannejad, H. Aghabozorg, Y. Mohammadzadeh, S. Hooshmand, Acta Crystallogr. E64 (2008) m870.
X.-M. Li, Q.-W. Wang, B. Liu, Acta Crystallogr. E63 (2007) m2443.
X.-M. Li, Y.-L. Niu, Q.-W. Wang, B. Liu, Acta Crystallogr. E63 (2007) m487.
D.-Q. Li, X. Liu, J. Zhou, Inorg. Chem. Comm. 11 (2008) 367.
H. Aghabozorg, P. Dalir Kheirollahi, A. Moghimi, E. Sadr-khanlou, Anal. Sci. 21 (2005) x79.
M.A. Sharif, H. Aghabozorg, A. Shokrollahi, G. Kickelbick, A. Moghimi, M. Shamsipur, Polish J. Chem. 80 (2006) 847.
L. Shimoni-Livny, J.P. Glusker, C.W. Bock, Inorg. Chem. 37 (1998) 1853.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Soleimannejad, J., Aghabozorg, H., Hooshmand, S. et al. Two novel metal organic frameworks of Sn(II) and Pb(II) with Pyridine-2,6-dicarboxylic Acid and 4,4′-Bipyridine: syntheses, crystal structures and solution studies. JICS 7, 405–418 (2010). https://doi.org/10.1007/BF03246026
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF03246026