Abstract
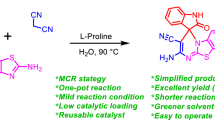
In this study we report a novel one-pot reaction which stereoselectively affords alkyl spiro[indeno[1,2-b]quinoxaline-11,3′- pyrrolizine]-2′-carboxylates via the four-component condensation of ninhydrin, phenylenediamines, proline, and acrylic acid derivatives. The reactions were run in ethanol and completed at less than 25 min and the products were obtained in very good yields. The stereochemistry of the products was deduced on the basis of the 1H NOESY and comparison with the related systems.
Similar content being viewed by others
References
For a monograph, see: J. Zhu, H. Bienayme, Multicomponent reactions, VCH, Weinhein, Germany, 2005.
For reviews, see: a) A. Domling, I. Ugi, Angew. Chem. Int. Ed. 39 (2000) 3169; b) I. Ugi, S. Heck, Comb. Chem. High Throughput Screen 4 (2001) 1; c) L. Weber, Drug Discovery Today 7 (2002) 143; d) C. Hulme, V. Gore, Curr. Med. Chem. 10 (2003) 51; e) R.V.A Orru, M. de Greef, Synthesis (2003) 1471; f) D.J. Ramon, M. Yus, Angew. Chem. Int. Ed. 44 (2005) 1602; g) M. Syamala, Org. Prep. Proced. Int. 37 (2005) 103; h) A. Domling, Chem. Rev. 106 (2006) 17.
For selected recent examples, see: a) C. Montagne, J.J. Shiers, M. Shipman, Tetrahedron Lett. 47 (2006) 9207; b) M.S.A. Dondoni, A. Massi, Acc. Chem. Res. 39 (2006) 451; c) P. Toto, J.C. Gesquiere, N. Cousaert, B. Deprez, N. Willand, Tetrahedron Lett. 47 (2006) 4973; d) T. Ngouansavanh, J.P. Zhu, Angew. Chem., Int. Ed. 45 (2006) 3495; e) J. Pospisil, T. Kumamoto, I.E. Marko, Angew. Chem., Int. Ed. 45 (2006) 3357; f) G. Sklute, I.J. Marek, J. Am. Chem. Soc. 128 (2006) 4642.
A. Gazit, H. App, G. Mc Mahon, J. Chen, A. Levitzki, F.D. Bohmer, J. Med. Chem. 39 (1996) 2170.
U. Sehlstedt, P. Aich, J. Bergman, E.I. Vallberg, B. Norden, A. Graslund, J. Mol. Biol. 278 (1998) 3156
R.P. Trillo, Univ. Microfilms (Ann. Arbor. Mich.), L.C. card no. Mic. 59-4672, 106, Dissertation Abstr. 20 (1959) 1597.
S. Tries, S. Laufer, Inflammopharmacology 9 (2001) 113
N.G. Argyropoulos, V.C. Sarli, M. Gdaniec, Eur. J. Org. Chem. (2006) 3738
E. Borsini, G. Broggini, A. Contini, G. Zecchi, Eur. J. Org. Chem. (2008) 2808, and references cited there in
H. Farsam, N. Yassa, P. Sarkhail, A. Shafiee, Planta Med. 66 (2000) 389
N. Yassa, H. Farsam, A. Shafiee, A. Rustaiyan, Planta Med. 62 (1996) 583
H. Farsam, N. Yassa, A. Shafiee, M. Amanlou, M. Biglar, T. Pourlotfali, Pharm. Pharmacol. Lett. 4 (1998) 79
N. Yassa, H. Farsam, A. Rustaiyan, A. Shafiee, J. Sci. I.R. Iran 10 (1999) 39.
W.J. Lown, in: A. Padwa (Ed.), 1,3-Dipolar Cycloaddition Chemistry, Vol. 1, Wiley, New York, 1984, p. 653
R. Huisgen, A. Padwa (Eds.), 1,3-Dipolar Cycloaddition Chemistry, Vol. 1, Wiley, New York, 1984, p. 1
A. Padwa (Ed.), 1,3-Dipolar Cycloaddition Chemistry, Vol. 1, Wiley, New York, 1984, p. 227
E. Vedejs, in: D.P. Curran (Ed.), Advances in Cycloadditions, Vol. 1, JAI Press, Greenwich, CT, 1988, p. 33
O. Tsuge, S. Kanemasa, Adv. Heterocycl. Chem. 45 (1989) 231
A. Padwa, in: B.M. Trost, I. Fleming (Eds.), Comprehensive Organic Synthesis, Vol. 4, Pergamon Press, Oxford, 1991, p. 1069
P.A. Wade, in: B.M. Trost, I. Fleming (Eds.), Comprehensive Organic Synthesis, Vol. 4, Pergamon Press, Oxford, 1991, p. 1111.
K.V. Gothelf, K.A. Jørgensen, Chem. Rev. 98 (1998) 863
C. Najera, G.M. Curr. Org. Chem. 7 (2003) 1105.
J. Azizian, M.R. Mohammadizadeh, S. Zomorodbakhsh, A.A. Mohammad, A.R. Karimi, Arkivoc XV (2007) 25
J. Azizian, M.R. Mohammadizadeh, S. Zomorodbakhsh, A.A. Mohammad, A.R. Karimi, Heteroatom Chem. 16 (2005) 259
J. Azizian, M.R. Mohammadizadeh, A.A. Mohammad, A.R. Karimi, J. Org. Chem. 70 (2005) 350
J. Azizian, A.R. Karimi, Z. Kazemizadeh, A.A. Mohammad, M.R. Mohammadizadeh, Synthesis (2005) 1095
J. Azizian, A.R. Karimi, Z. Kazemizadeh, A.A. Mohammad, M.R. Mohammadizadeh, J. Org. Chem. 70 (2005) 1471.
J. Azizian, M.R. Mohammadizadeh, N. Karimi, Z. Kazemizadeh, A.A. Mohammad, A.R. Karimi, Heteroatom Chem. 16 (2005) 549
J. Azizian, M.R. Mohammadizadeh, A.A. Mohammad, A.R. Karimi, F. Teimouri, Heteroatom Chem. 18 (2007) 16
J. Azizian, A.R. Karimi, A.A. Mohammad, M.R. Mohammadizadeh, Synthesis (2004) 2263.
P. Allway, R. Grigg, Tetrahedron Lett. 32 (1991) 5817
T. Coulter, R. Grigg, J.F. Malone, V. Sridharan, Tetrahedron Lett. 32 (1991) 5417
R. Grigg, J. Idle, M. McMeekin, S. Surendrakumar, D. Vinod, J. Chem. Soc., Perkin Trans. 1 (1988) 2693
R. Grigg, J. Idle, M. McMeekin, S. Surendrakumar, D. Vinod, J. Chem. Soc., Perkin Trans. 1 (1988) 2703.
M. Friedman, J. Agric. Food Chem. 52 (2004) 385
J.L. Hallman, R.A. Bartsch, J. Org. Chem. 56 (1991) 6243
D.J. McCaldin, Chem. Rev. 60 (1960) 39
R. Shapiro, N. Chatterjie, J. Org. Chem. 35 (1970) 447
G.H. Posner, Chem. Rev. 86 (1986) 831.
G. Broggini, G. Zecchi, Synthesis (1999) 905
C. Najera, J.M. Sansano, Angew. Chem. Int. Ed. Engl. 44 (2005) 6272.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Karsalary, A.A., Mohammadizadeh, M.R., Hasaninejad, A.R. et al. A novel, fast and efficient one-pot four-component procedure for preparation of some alkyl spiro[indeno[1,2-b]quinoxaline-11,3′-pyrrolizine]-2′-carboxylates. JICS 7, 45–50 (2010). https://doi.org/10.1007/BF03245858
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF03245858