Abstract
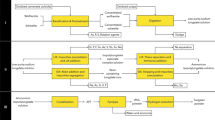
This article describes the conversion of arsenic trioxide into crystalline scorodite (FeAsO4 • 2H2O) using acidic nitrate solutions at temperatures ranging from 140 °C to 160 °C. A schematic process flow sheet is proposed. The reasons for processing arsenic trioxide, especially baghouse dust from metallurgical operations, lie in its toxic character and the fact that market demand for it is decreasing. An environmentally friendly way to dispose of this roaster by-product is as crystalline ferric arsenate (scorodite), since the latter has been shown to be the least soluble “host” mineral for arsenic. To produce crystalline scorodite in bulk has to date not been possible, however. This article describes a new hydrometallurgical approach for converting arsenic trioxide rapidly and at a high yield into crystalline scorodite. It is based on the fact that at 160 °C, ferric does not precipitate as an oxide from solutions that contain 2 M HNO3. By adding arsenic trioxide to such a solution, ferric precipitates as crystalline scorodite, without other iron species (e.g., hematite) precipitating. This means that a stoichiometric Fe:As ratio is sufficient. When starting with arsenic trioxide, trivalent arsenic will be oxidized before 160 °C is reached due to the strong oxidizing power of nitric acid.
Similar content being viewed by others
References
J.E. Hoffmann, “Remediating Copper Smelter Dusts: The Arsenic Problem,” JOM, 45 (August 1993), pp. 30–31.
Shell Company News Release on PLATO Process in Shell Venster, Rotterdam (November 1993).
E. Krause and E.V. Ettel, “Solubilities and Stabilities of Ferric Arsenate Compounds,” Hydrometallurgy, 22 (1989), pp. 311–337.
Y. Shang and G. Van Weert, “Iron Control in Nitrate Hydrometallurgy by Autoclave Hydrolysis of Ferric Nitrate,” Hydro-metallurgy, 33 (1993), pp. 273–290.
R.G. Robins, “Arsenic Hydrometallurgy, ”Arsenic Metallurgy Fundamentals and Applications, ed. R.G. Reddy, J.L. Hendrix, and P.B. Queneau (Warrendale, PA: TMS, 1988), pp. 215–247.
DJ. Droppert and G. Van Weert, “ Arsenic Trioxide Conversion to Crystalline Scorodite Using Nitrate Hydrometallurgy,}” Billiton-sponsored study.
G.B. Harris and S. Monette, “The Stability of Arsenic Bearing Residues,” Arsenic Metallurgy Fundamentals and Applications, ed. R.G. Reddy, J.L. Hendrix, and P.B. Queneau (Warrendale, PA: TMS, 1988), pp. 469–488.
M. Stefanakis and A. Kontopoulos, “Production of Environmentally Acceptable Arsenites—Arsenites from Solid Arsenic Trioxide,” Arsenic Metallurgy Fundamentals and Applications, ed. R.G. Reddy, J.L. Hendrix, and P.B. Queneau (Warrendale, PA: TMS, 1988), pp. 287–304.
C.G. Anderson, K.D. Harrison, and L.E. Krys, “Process Integration of Sodium Nitrite Oxidation and Fine Grinding in Refractory Precious Metal Concentrate Pressure Leaching” (Paper presented at the SME Annual Meeting, Albuquerque, NM, February} 1994, available as preprint no. 94-45).
G. Van Weert and R.M.D. Rodermond, “Selective Nickel and Cobalt Solubilization from Limonitic Ores in Acidic Nitrate Media” (Paper to be presented at the 24th Annual CIM Hydrometallurgical Meeting in Toronto, Canada, August 1994).
G. Van Weert and V. Kok, “Impurity Levels in Hematite Produced by Autoclave Hydrolysis of Ferric Nitrate” (Paper to be presented at the 1995 TMS Annual Meeting, Las Vegas, NV, 12–16 February} 1995).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Van Weert, G., Droppert, D.J. Aqueous processing of arsenic trioxide to crystalline scorodite. JOM 46, 36–38 (1994). https://doi.org/10.1007/BF03220716
Issue Date:
DOI: https://doi.org/10.1007/BF03220716