Abstract
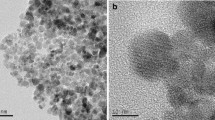
The monodisperse chitosan-coated Fe3O4 nanoparticles with a mean diameter of 13.5 nm and 4.92 wt% chitosan were used as an anionic magnetic nano-adsorbent for the recovery of Au(III) ions from aqueous solutions. It was found that Au(III) ions could be fast and efficiently adsorbed, and the adsorption capacity increased with the decrease in pH due to the protonation of the amino groups of chitosan. The adsorption data obeyed the Langmuir equation with a maximum adsorption capacity of 59.52 mg/g (1210 mg/g based on the weight of chitosan) and a Langmuir adsorption equilibrium constant of 0.066 l/mg. From the studies on the adsorption kinetics and thermodynamics of Au(III) ions, it was found that the adsorption process obeyed the pseudo-second-order kinetic model. Furthermore, the time required to reach the equilibrium was significantly shorter than those using the micro-sized adsorbents due to the large available surface area.
Article PDF
Similar content being viewed by others
Avoid common mistakes on your manuscript.
References
I. Villaescusa, V. Salvadó, J. de Pablo,Hydrometallurgy, 1996,41, 303
M.A. Barroso, F.A. López, A.M. Saatre, F.J. Alguacil,Hydrometallurgy, 1996,45, 199
C.P. Gomes, M.F. Almeida, J.M. Loureiro,Sep. Purif. Technol., 2001,24, 35
E. Anticó, A. Masana, V. Salvadó, M. Hidalgo, M. Valiente,Anal. Chim. Acta, 1984,296, 325
M. Iglesias, E. Anticó, V. Salvadó,Anal. Chim. Acta, 1999,381, 61
M.J. Alonso,Biomed. & Pharmacother., 2004,58, 168
A. Bozkir, O.M. Saka,Drug Deliv., 2004,11, 107
A.K. Singla, M. Chawla,J. Pharm. Pharmacol., 2001,53, 1047
J.L. Chew, C.B. Wolfowicz, H.Q. Mao, K.W. Leong, K.Y. Chua,Vaccine, 2003,21, 2720
X.G. Chen, C.M. Lee, H.J. Park,J. Agr. Food Chem., 2003,51, 3135
M. Kabbaj, N.C. Phillips,J. Drug Target., 2001,9, 317
G. Karthikeyan, K. Anbalagan, N.M. Andal,J. Chem. Sci., 2004,116, 119
R.S. Juang, H.J. Shao,Water Res., 2002,36, 2999
J.C.Y. Ng, W.H. Cheung, G. McKay,J. Colloid Interface Sci., 2002,255, 64
K. Inoue, K. Yoshizuka, K. Ohto,Anal. Chem. Acta, 1999,388, 209
M.L. Arrascue, H.M. Garcia, O. Horna, E. Guibal,Hydrometallurgy, 2003,71, 191
M. Ruiz, A.M. Sastre, E. Guibal,React. Funct. Polym., 2000,45, 155
T.Y. Hsien, G.L. Rorrer,Sep. Sci. Technol., 1995,30, 2455
T.Y. Hsien, G.L. Rorrer,Ind. Eng. Chem. Res., 1997,36, 3631
A.L. Debbaudt, M.L. Ferreira, M.E. Gschaider,Carbohydr. Polym., 2004,56, 321
M.W. Wan, I.G. Petrisor, H.T. Lai, D. Kim, T.F. Yen,Carbohydr. Polym., 2004,55, 249
Y.C. Chang, D.H. Chen,J. Colloid Interface Sci., 2005,283, 446
Y.C. Chang, D.H. Chen,Macromol. Biosci., 2005,5, 254
Y.C. Chang, D.B. Shieh, C.H. Chang, D.H. Chen,J. Biomed. Nanotechnol., 2005,1, 196
W.S.W. Ngah, K.H. Liang,Ind. Eng. Chem. Res., 1999,38, 1411
E. Tutem, R. Apak, G.F. Unal,Water Res., 1998,32, 2315
Y.S. Ho, G. McKay,Process Biochem., 1999,34, 451
W.J. Weber Jr., J.C. Morriss, J. Sanitary,Eng. Div. Am. Soc. Civ. Eng., 1963,89, 31
R.S. Juang, F.C. Wu, R.L. Tseng,Environ. Technol., 1997,18, 525
W. Stumm, J.J. Morgan,Aquatic Chemistry: An Introduction Emphasizing Chemical Equilibria in Natural Waters, 2nd edn, J. Wiley & Sons, New York, 1981
H. Nollet, M. Roels, P. Lutgen, P. Van der Meeren, W. Verstrate,Chemosphere, 2003,53, 655
Author information
Authors and Affiliations
Corresponding author
Additional information
Dr. Yang-Chuang Chang received his PhD in Chemical Engineering at National Cheng Kung University (Taiwan), focusing on the fabrication and applications of multifunctional magnetic nano-carriers. He currently is a researcher at Institute of Nuclear Energy Research (Taiwan), working on the development of fuel cells.
Dr. Dong-Hwang Chen is a professor of Chemical Engineering Department at National Cheng Kung University (Taiwan). His research works include the synthesis of nanoparticles, the fabrication of functional composite nanoparticles and thin films, the applications of surfacemodified magnetic nanoparticles in separation, catalysis, and biomedicine, as well as the development of composite nanomaterials for electromagnetic wave absorption.
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License ( https://creativecommons.org/licenses/by-nc/2.0 ), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Chang, YC., Chen, DH. Recovery of gold(III) ions by a chitosancoated magnetic nano-adsorbent. Gold Bull 39, 98–102 (2006). https://doi.org/10.1007/BF03215536
Issue Date:
DOI: https://doi.org/10.1007/BF03215536