Abstract
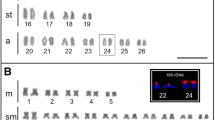
Employing FISH analysis as well as BLAST and CUSTAL W (1.82) programs, we investigated types of DNA nucleotide sequences building an additional heterochromatic band in 2R chromosomes of 3 lines ofSecale vavilovii Grossh. The probes used in FISH analysis were designed based on the reverse transcriptase sequence of Ty1-copia and Ty3-gypsy retrotransposons and the 5S rRNA gene sequence. No hybridization signals from the reverse transcriptase probes were observed in the chromosome region where the additional band occurs. On the other hand, signals were observed after hybridization with the 5S rDNA probe, clearly suggesting the presence of that type of sequences in the analyzed heterochromatin band. Using BLAST and CUSTAL W programs, we revealed high similarity of the JNK1 sequence to the 5S rRNA gene fromHordeum chilense (HCH1016, HCH1018, 88%) and to a fragment of the 5S rRNA sequence ofH. marinum (HMAR003, 97%). In addition, the same fragment of JNK1 was shown to be very similar to the part of theAngela retrotransposon (92%) as well as to theSNAC 426K20-1 transposon (89%) belonging to CACTA family, both fromTriticum monococcum, and toZingeria biebersteiniana pericentromeric sequences (78%). The similarity of JNK1 to those sequences may be accidental or the JNK1 may represent an ancient mobile genetic element that caught the 5S rRNA sequence. During the evolution those sequences might have been accumulated in the particular region on the 2R chromosome. Our results suggest that the additional heterochromatin band in chromosomes 2R ofS. vavilovii is a collection of defective genes and/or mobile genetic elements.
Similar content being viewed by others
References
Achrem M, Kalinka A, Rogalska SM, 2005. Localization of the gene coding the transposase of maize Ac/Ds system on rye chromosomes (Secale vavilovii Grossh. andS. cereale L.) by FISH. In: Variability and evolution: new perspectives, W. Prus-Głowacki, E. Pawlaczyk, eds. 499–505; Adam Mickiewicz University Press, Poznań.
Adams MD, Celniker SE, Holt RA, Evans CA, Gocayne JD, et al. 2000. The genome sequence ofDrosophila melanogaster. Science 287: 2185–2195.
Almeida K, Allshire RC, 2005. RNA silencing and genome regulation. Trends Cell Biol 15: 251–258.
Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ, 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25: 3389–3402.
Appels R, Driscoll C, Peacock WJ, 1978. Heterochromatin and highly repeated DNA sequences in rye (Secale cereale). Chromosoma 70: 67–89.
Bedbrook JR, Jones J, O’DellM, Thompson RD, Flavell RB, 1980. A molecular description telomeric heterochromatin inSecale species. Cell 19: 545–560.
Bennett MD, Gustafson JP, Smith JB, 1977. Variation in nuclear DNA in the genusSecale. Chromosoma 61: 149–176.
Bennetzen JL, 2000. Transposable element contributions to plant gene and genome evolution. Plant Molecular Biology 42: 251–269.
Chopra S, Brendel V, Zhang J, Axtell JD, Peterson T, 1999. Molecular characterization of a mutable pigmentation phenotype and isolation of the first active transposable element fromSorghum bicolor. Proc Natl Acad Sci USA 96: 15330–15335.
Csink K, Henikoff S, 1998. Something from nothing: the evolution and utility of satellite repeats. Trends Genet. 14: 200–204.
Dimitri P, Junakovic N, 1999. Revising the selfish DNA hypothesis: new evidence on accumulation of transposable elements in heterochromatin. Trends Genet. 15: 123–124.
Douet J, Tourmente S, 2007. Transcription of the 5S rRNA heterochromatic genes is epigenetically controlled inArabidopsis thaliana andXenopus laevis. Heredity 99: 5–13.
Dover GA, 1986. Molecular drive in multigene families: how biological novelties arise, spread and are assimilated. Trends Genet 2: 159–165.
Feuillet C, Penger A, Gellner K, Mast A, Keller B, 2001. Molecular evolution of receptor-like kinase genes in hexaploid wheat: independ entevolution of orthologs after polyploidization and mechanisms of local rearrangements at paralogous loci. Plant Physiol 125: 1304–1313.
Flavell RB, 1986. Repetitive DNA and chromosome evolution in plants. Philos. Trans. R. Soc. Lond. B Biol. Sci. 312: 227–242.
Flavell AJ, Smith DB, Kumar A, 1992. Extreme heterogeneity of Ty1-copia group retrotransposons in plants. Mol Gen Genet. 231: 233–242.
Friesen N, Brandes A, Heslop-Harrison J, 2001. Diversity, origin and distribution of retrotransposons in conifers. Mol Biol Evol 18: 1176–1188.
Fukui KN, Suzuki G, Lagudah ES, Rahman S, Appels R, Yamamoto M, Mukai Y, 2001. Physical arrangement of retrotransposon-related repeats in centromeric regions of wheat. Plant Cell Physiol 42: 189–196.
Gill B, Kimber G, 1974. The Giemsa-C-banded karyotype of rye. Proc Nat Acad Sci USA 71: 1247–1249.
Hancock JM, 1996. Simple sequences and the expanding genome. BioEssays 18: 421–425.
Heslop-Harrison JS, Brandes A, Taketa S, 1997. The chromosomal distribution of Ty1-copia group retrotransposable elements in higher plants and their implications for genome evolution. Genetica 100: 197–204.
Jiang N, Bao Z, Zhang X, Eddy SR, Wessler SR, 2004. Pack-MULE transposable elements mediate gene evolution in plants. Nature 431: 569–573.
Kalendar R, Tanskanen J, Chang W, Antonius K, Sela H, Peleg O, Schulman AH, 2008.Cassandra retrotransposons carry independently transcribed 5S RNA. Proc Natl Acad Sci USA 105: 5833–5838.
Lee JK, Kwon SJ, Park KC, Kim NS, 2005.Isaac-CACTA transposons: new genetic markers in maize and sorghum. Genome 48: 455–460.
Lewin B, 1997. Transposons. In: Genes VI. Oxford University Press, Inc., New York B. Lewin, ed. 563–595.
Martienssen R, Moazed D, 2006. RNAi and heterochromatin assembly. CSHL Epigenetics text-book. D. Allis, T. Jenuwein, D. Reinberg, eds. Cold Spring Harbor Laboratory Press, NY, USA.
Miura A, Yonebayashi S, Watanabe K, Toyama T, Shimada H, Kakutani T, 2001. Mobilization of transposons by a mutation abolishing full DNA methylation inArabidopsis. Nature 411: 212–214.
Morgante M, Brunner S, Pea G, Fengler K, Zuccolo A, Rafalski A, 2005. Gene duplication and exon shuffling by helitron-like transposons generate intraspecies diversity in maize. Nat Genet 37: 997–1002.
Murray M, Thompson WF, 1980. Rapid isolation of molecular Wright plant DNA. Nucleic Acids Research 8: 4321–4325.
Nacken WKF, Piotrowiak R, Saedler H, Sommer H, 1991. The transposable elementTAM-1 ofAntirrhinum majus shows structural homology to the maize transposonEn/Spm and has no sequence specificity of insertion. Mol Gen Genet 228: 201–208.
Nagaki K, Tsujimoto H, Saskuma T, 1999. A novel repetitive sequence, termedthe JNK repeat family, located on an extra heterochromatic region of chromosome 2R of Japanese rye. Chromosome Research 6: 95–101.
Ozeki Y, Davies E, Takeda J, 1997. Somatic variation during long term subculturing of plant cells caused by insertion of a transposable element in a pheny lalanine ammonia-lyase(PAL) gene. MolGen Genet 254: 407–416.
Reddy P, Appels R, 1989. A second locus for the 5S multigene family inSecale cereale L. sequence divergence in two lineages of the family. Genome 32: 457–467.
Redi CA, Garagna S, Zacharias H, Zuccotti M, Capanna E, 2001. The other chromatin. Chromosoma 110: 136–147.
Rogalska SM, 1978. Rozmieszczenie heterochromatyny w chromosomach kilku odmian diploidalnego żytaSecale cereale L. [Distribution of heterochromatin in chromosomes of several cultivars ryeSecale cereale L.]. Hodowla Roślin 5: 7–10 (in Polish).
Rogalska SM, 1992. Charakterystyka molekularna i cytogenetyczna heterochromatyny w chromosomach żyta (Secale cereale L.). [Molecular characteristics and cytogenetic role of heterochromatin in chromosomes of rye (Secale cereale L.)]. Post Biol Kom 19: 107–116 (in Polish).
Rogalska S, Apolinarska B, 1998. Mobility of C-band on chromosomes of ryeSecale vavilovii. Cytogenetics and Cell Genetics, Proceedings 13th International Chromosome Conference Ancona (Italy): 145.
Rogalska SM, Achrem M, Stróżycki P, 2001. The pScJNK1 repeated sequences identified on an extra heterochromatin bandon chromosome 2R ofSecale vavilovii Grossh. lines. Biological Bulletin 38: 15–19.
Rogalska SM, Achrem M, Słomińska-Walkowiak R, Filip E, Skuza L, Pawłowska J, Apolinarska B, 2002. Polymorphism of heterochromatin bands on chromosomes of ryeSecale vavilovii Grossh. lines. Acta Biol Crac ser. Botanica 44: 111–117.
Rogalska S, Achrem M, Kalinka A, 2007. Occurrence of JNK sequences in the species of the genusSecale. Vorträge für Pflanzenzüchtung 71: 181–188.
Rogers SO, Bendich AJ, 1987. Ribosomal RNA genes in plants: variability in copy number and in the intergenic spacer. Plant Molecular Biology 9: 509–520.
Saunders VA, Houben A, 2001. The pericentromeric heterochromatin of the grassZingeria biebersteiniana (2n = 4) is composed of Zbcen1-type tandem repeats that are intermingled with accumulated dispersedly organized sequences. Genome 44: 955–961.
Shang HY, Baum B, Wei YM, Zheng YL, 2007. The 5SrRNA gene diversity in the genusSecale and determination of its closest haplomes. Genet Res Crop Evol 54: 793–806.
Snowden KC, Napoli CA, 1998.PsI: a novel Spm-like transposable element fromPetunia hybrida. Plant J 14: 43–54.
Strand M, Prolla TA, Liskay RM, Petes TD, 1993. Destabilization of tracts of simple repetitive DNA in yeast by mutations affecting DNA mismatch repair. Nature 365: 274–276.
Tessadori F, Chupeau M-C, Chupeau Y, Knip M, Germann S, Driel R, et al. 2007. Large-scale dissociation and sequential reassembly of pericentric heterochromatin in dedifferentiatedArabidopsis cells. Journal of Cell Science 120: 1200–1208.
Thompson J, Higgiin D, Gibson T, 1994. CLUSTALW: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position specific gap penalties and weight matrix choice. Nuc Acids Res, 22: 4673–4680.
Turner BM, 2001. Chromatin and gene regulation: molecular mechanisms in epigenetics. Blackwell Science, Oxford.
Vershinin AV, Schwarzacher T, Heslop-Harrison JS, 1995. The large-scale genomic organization of repetitive DNA families at the telomeres of rye chromosomes. Plant Cell 7: 1823–1833.
Wicker T, Gujot R, Yahiaoui N, Keller B, 2000 3. CACTA transposons in Triticeae: diverse family of high-copy repetitive elements. Plant Physiol 132: 52–63.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Achrem, M., Rogalska, S.M. & Kalinka, A. Possible ancient origin of heterochromatic JNK sequences in chromosomes 2R ofSecale vavilovii Grossh. J Appl Genet 51, 1–8 (2010). https://doi.org/10.1007/BF03195704
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF03195704