Abstract
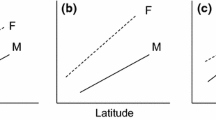
We studied the intra- and interspecific size variability of 271 water shrewsNeomys fodiens (Pennant, 1771) andN. anomalus Cabrera, 1907 from seven sample sites along a latitudinal transect from Bosnia and Herzegovina to Poland.Neomys anomalus was the only water shrew in three Dinaride karst fields, while it was sympatric with N.fodiens in remaining sites. The first principal component scores (PC1; 72.2% of variance explained), derived from principal components analysis of 13 cranial, mandibular and dental measurements, were used as the size factor. One-way ANOVA detected significant interpopulation variation in both species; intraspecific variation, however, was much more pronounced inN. anomalus. No latitudinal size pattern was found in N. fodiens (r = −0.42, p = 0.58), while mean PC1 scores correlated significantly and negatively with latitude inN. anomalus (r = −0.92, p = 0.004). Therefore, along a north to south transect,N. anomalus converged in size towards N. fodiens, which suggests that the former species occupies increasingly more aquatic habitats in the same direction. Individuals from allopatric populations ofN. anomalus from Slovenia and Bosnia and Herzegovina were, on average, larger than sympatric conspecific populations from the same latitudinal zone, which is consistent with the hypothesis of character displacement.
Similar content being viewed by others
References
Adams D. C. and Rohlf F. J. 2000. Ecological character displacement inPlethodon: biomechanical differences found from a geometric morphometric study. Proceedings of the National Academy of Science 97: 4106–4111.
Aitchison C. W. 1987. Review of winter trophic relations of soricine shrews. Mammal Review 17: 1–24.
Ashton K. G. 2002. Patterns of within-species body size variation of birds: strong evidence for Bergmann’s rule. Global Ecology and Biogeography 11: 505–523.
Bergmann C. 1847. Ueber die Verhältnisse der Wärmekönomie der Thiere zu ihrer Grösse. Gottinger Studien 3: 595–708.
Bonacci O. 2004. Poljes. [In: Encyclopedia of caves and karst science. J. Gunn, ed]. Fitzroy Dearborn, New York: 599–600.
Boyce M. S. 1978. Climatic variability and body size variation in the muskrats (Ondatra zibethicus) of North America. Oecologia 36: 1–19.
Brown W. L. and Wilson E. O. 1956. Character displacement. Systematic Zoology 5: 49–64.
Calder W. A. 1984. Size, function, and life history. Harvard University Press, Cambridge: 1–431.
Carraway L. N. and Verts B. J. 2005. Assessment of variation in cranial and mandibular dimensions in geographical races ofSorex towbridgii. [In: Advances in the biology of shrews II. J. F. Merritt, S. Churchfield, R. Hutterrer and B. I. Sheftel, eds]. International Society of Shrew Biologists, New York: 139–153.
Churchfield S. 1990. The natural history of shrews. Christopher Helm, London: 1–178.
Churchfield S. and Rychlik L. 2006. Diets and coexistence inNeomys andSorex shrews in Białowieża forest, eastern Poland. Journal of Zoology, London 269: 381–390.
Churchfield S., Rychlik L., Yavrouyan E. and Turlejski K. 2006. First results on the feeding ecology of the Transcaucasian water shrewNeomys teres (Soricomorpha: Soricidae) from Armenia. Canadian Journal of Zoology 84: 1853–1858.
Dickman C. R. 1988. Body size, prey size, and community structure in insectivorous mammals. Ecology 69: 569–580.
Dickman C. R. 1991. Mechanisms of competition among insectivorous mammals. Oecologia 85: 464–471.
Doebeli M. 1996. An explicit genetic model for ecological character displacement. Ecology 77: 510–520.
Findley J. S. 1989. Morphological patterns in rodent communities of soutwestern North America. [In: Patterns in the structure of mammalian communities. D. W. Morris, Z. Abramsky, B. J. Fox and M. R. Willig, eds]. Texas Tech University Press, Lubbock: 253–263.
Fordyce J. A. 2006. The evolutionary consequences of ecological interactions mediated through phenotypic plasticity. The Journal of Experimental Biology 209: 2377–2383.
Geist V. 1987. Bergmann’s rule is invalid. Canadian Journal of Zoology 65: 1935–1038.
Grant P. R. and Grant B. R. 2006. Evolution of character displacement in Darwin’s finches. Science 313: 224–226.
Hanski I. and Kaikusalo A. 1989. Distribution and habitat selection of shrews in Finland. Annales Zoologici Fennici 26: 339–348.
Hutterer R. 1985. Anatomical adaptations of shrews. Mammal Review 15: 43–55.
Kendeight S. C. 1969. Tolerance of cold and Bergmann’s rule. Auk 86: 13–25.
Krushinska N. L. and Pucek Z. 1989. Ethological study of sympatric species of European water shrews. Acta Theriologica 34: 269–285.
Krushinska N. L. and Rychlik L. 1993. Intra- and interspecific antagonistic behavious in two sympatric species of water shrews:Neomys fodiens andN. anomalus. Journal of Ethology 11: 11–21.
Kryštufek B., Davison A. and Griffiths H. I. 2000. Evolutionary beiogeography of water shrews (Neomys spp.) in the western Palaearctic region. Canadian Journal of Zoology 78: 1616–1625.
Lemen C. A. 1983. The effectiveness of methods of shape analysis. Fieldiana Zoology N.S. 15: 1–17.
Lindstedt S. L. and Boyce M. S. 1985. Seasonality, fasting endurance, and body size in mammals. The American Naturalist 125: 873–878.
Mayr E. 1963. Animal species and evolution. Harvard University Press, Cambridge, MA: 1–797.
McNab B. K. 1971. On the ecological significance of Bergmann’s rule. Ecology 52: 845–854.
McNab B. K. 2006. The evolution of energetics in eutherian “insectivorans”: an alternate approach. Acta Theriologica 51: 113–128.
Meiri S. and Dayan T. 2003. On the validity of Bergmann’s rule. Journal of Biogeography 30: 331–351.
Mitchell-Jones A., Amori G., Bogdanowicz W., Kryštufek B., Reijnders P. J. H., Spitzenberger F., Stubbe M., Thissen J. B. M., Vohralik V. and Zima J. 1999. The atlas of European mammals. Poyser Natural History, London: 1–484.
Niethammer J. and Krapp F. 1990. Handbuch der Säugetiere Europas. Band 3/I: Insektenfresser, Herrentiere. Aula-Verlag, Wiesbaden: 1–523.
Ochocińska D. and Taylor J. R. E. 2003. Bergmann’s rule in shrews: geographical variation of body size in Palearctic Sorex species. Biological Journal of the Linnean Society 78: 365–381.
Pfenning D. W., Rice A. M. and Martin R. A. 2006. Ecological opportunity and phenotypic plasticity interact to promote character displacement and species coexistence. Ecology 87: 769–779.
Pucek Z. 1964. The structure of the glans penis inNeomys Kaup, 1929 as a taxonomic character. Acta Theriologica 9: 374–377.
Rácz G. and Demeter A. 1998. Character displacement in mandible shape and size in two species of water shrews (Neomys, Mammalia: Insectivora). Acta Zoologica Academiae Scientarum Hungaricae 44: 165–175.
Rychlik L., Ramalhinho G. and Polly D. 2006. Response to environmental factors and competition: skull, mandible, and tooth shape in Polish water shrews (Neomys, Soricidae, Mammalia). Journal of Zoological Systematics and Evolutionary Research 44: 339–351.
Schluter D. 2000. Ecological character displacement and adaptive radiation. The American Naturalist 156: S4-S16.
Schluter D. and McPhail J. D. 1992. Ecological character displacement and speciation in sticklebacks. The American Naturalist 140: 85–108.
Snell R. R. and Cunnison K. M. 1983. Relation of geographic variation in the skull ofMicrotus pennsylvanicus to climate. Canadian Journal of Zoology 61: 1232–1241.
Sneth P. H. A. and Sokal R. R. 1973. Numerical taxonomy: the principles and practice of numerical classification. Freeman and Company, San Francisco: 1–962.
Taper M. L. and Case T. J. 1992. Coevolution among competitors. Oxford Surveys in Evolutionary Biology 86: 63–109.
White T. A. and Searle J. B. 2007. Factors explaining increased body size in common shrews (Sorex araneus) on Scottish islands. Journal of Biogeography 34: 356–363.
Wolff J. O. and Guthrie R. D. 1985. Why are aquatic small mammals so large? Oikos 45: 365–373.
Yom-Tov Y. and Geffen E. 2006. Geographic variation in body size: the effects of ambient temperature and precipitation. Oecologia 148: 213–218.
Yom-Tov Y. and Yom-Tov J. 2005. Global warming, Bergmann’s rule and body size in the masked shrewSorex cinereus Kerr in Alaska. Journal of Animal Ecology 74: 803–808.
Author information
Authors and Affiliations
Additional information
Associate editor was P. David Polly.
Rights and permissions
About this article
Cite this article
Kryštufek, B., Quadracci, A. Effects of latitude and allopatry on body size variation in European water shrews. Acta Theriol 53, 39–46 (2008). https://doi.org/10.1007/BF03194277
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF03194277