Summary
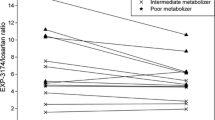
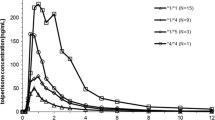
The contribution of the enzymes CYP2D6 and CYP2C19 to the metabolism of artemether was evaluated in a cross-over study in seven healthy adult Caucasian subjects. The pharmacokinetic properties of artemether and its active metabolite dihydroartemisinin were compared when given 100 mg artemether orally alone or in combination with either CYP2D6-inhibitor quinidine or CYP2C19-inhibitor omeprazole. Plasma concentrations of artemether and dihydroartemisinin were measured with reversed phase high performance liquid chromatography with electro-chemical detection (HPLC-ED). Artemether was rapidly absorbed with a mean tmax of 0.8 h (95% confidence interval, CI=0.5–1.1) reaching a mean Cmax of 29 ng/ml (14–45 ng/ml). The mean elimination half-life was 1.3 h (0.8–1.8 h). The pharmacokinetic parameters for dihydroartemisinin were not significantly different from those for artemether. Artemether combined with quinidine revealed no significant changes in the plasma concentrations of either artemether or dihydroartemisinin. No changes were seen in the combination with omeprazole as a CYP2C19 inhibitor. A second peak in the plasma concentration profile was observed 2–4 h after drug intake. This phenomenon was possibly related to variable gastric emptying. No major contribution of the enzymes CYP2D6 or CYP2C19 was found in artemether metabolism. No interethnic differences in artemether metabolism on the basis of a genetic polymorphism of these enzymes is to be expected.
Similar content being viewed by others
References
Wernsdorfer W. (1991): The development and spread of drug resistant malaria. Parasitol Today; 7; 297–303.
Hien T.T., White N.J. (1993): Qinghaosu. Lancet, 341, 603–608.
Klayman D. (1985): Qinghaosu (artemisinin): an antimalarial drug from China. Science, 228, 1049–1055.
De Vries P.J., Tran K.D. (1996): Clinical pharmacology and therapeutic potential of artemisinin and its derivatives in the treatment of malaria. Drugs, 52, 818–836.
Van Boxtel C.J., de Vries P.J., Tran K.D., Koopmans R.P., Kager P.A. (1995): Pharmacokinetics of artemisinin and derivatives. Proceedings of the 7th Southeast Asian/Western Pacific Regional Meeting of Pharmacologists. Manila, Philippines:Towards Rational Drug Therapy at the Turn of the Century, 498–504.
Van Boxtel C.J., van Agtmael M.A., de Vries P.J., Tran K.D., Koopmans R.P., Kager P.A. (1996): Some pharmacokinetic and dynamic comparisons of artemisinin derivatives in man. Jpn. J. Trop. Med. Hyg., 24, 4954.
Bangchang K., Karbwang J. (1994): Pharmacokinetics of artemether after oral administration to healthy Thai males and patients with acute, uncomplicated falciparum malaria. Br. J. Clin. Pharmacol., 37, 249–253.
Mordi M.N., Mansor S.M., Navaratnam V., Wernsdorfer W.H. (1997): Single dose pharmacokinetics of oral artemether in healthy Malaysian volunteers. Br. J. Clin. Pharmacol., 43, 363–365.
Lee I.S., Hufford C.D. (1990): Metabolism of antimalarial sesquiterpene lactones. Pharmacol. Ther., 48, 345–355.
Bertilsson L. (1995): Geographical/interracial differences in polymorphic drug oxidation, current state of knowledge of cytochromes P450 (CYP2D6 and 2C19). Clin. Pharmacokinet., 29, 192–209.
Tucker G.T. (1994): Clinical implications of genetic polymorphism in drug metabolism. J. Pharm. Pharmacol., 46, 417–424.
Kevin Park B., Munir Pirmohamed, Kitteringham N.R. (1995): The role of cytochrome P450 enzymes in hepatic and extrahepatic human drug toxicity. Pharmacol. Ther., 68, 385–424.
Brewer T.G., Peggins J.O., Grate S.J. et al., (1994): Neurotoxicity in animals due to arteether and artemether. Trans. R. Soc. Trop. Med. Hyg., 88 Suppl 1, 3336.
Zhou H.H., Anthony L.B., Roden D.M., Wood A.A.J. (1990): Quinidine reduces clearance of (+)-propranolol more than (−)-propranolol through marked reduction in 4-hydroxylation. Clin. Pharmacol. Ther.; 47, 686–693.
Speirs C.J., Murray S., Boobis A.R., Seddon C.E., Davies D.S. (1986): Quinidine and the identification of drugs whose elimination is impaired in subjects classified as poor metabolizers of debrisoquine. Br. J. Clin. Pharmacol., 22, 739–743.
Balian J.D., Sukhova N., Harris J.W. et al. (1995): The hydroxylation of omeprazole correlates with S-mephenytoin metabolism: a population study. Clin. Pharmacol. Ther., 57, 662–669.
Chang M., Tybring G., Dahl M.L. et al. (1995): Interphenotype differences in disposition on gastrin levels of omeprazole. Suitability of omeprazole as a probe for CYP2C19. Br. J. Clin. Pharmacol., 39, 511–518.
Caraco Y., Tateishi T., Wood A.J. (1995): Interethnic difference in omeprazole’s inhibition of diazepam metabolism. Clin. Pharmacol. Ther., 58, 62–72.
Melendez V., Peggins J.O., Brewer T.G., Theoharides A.D. (1991): Determination of the antimalarial arteether and its de-ethylated metabolite dihydroartemisinin in plasma by highperformance liquid chromatography with reductive electrochemical detection. J. Pharm. Sci., 80:132–138.
Van Agtmael M.A., Butter J.J., Portier E.J.G., Van Boxtel C.J. (1998): Validation of an improved reversed phase high performance liquid chromatography assay with reductive electrochemical detection for the determination of artemisinin derivatives in man. Ther. Drug Monit., 20, 109–116.
Scientist for experimental data fitting (1986–1995). Salt Lake City. Utah: Micromath Scientific Software.
Altman D.G. (1991): Comparing groups-continuous data. In: Practical statistics for medical research, 1st edn. London: Chapman & Hall.
Machin D., Campbell M.J., Fayers P.M., Pinol A.P.Y. (1987, 1997): Sample size tables for clinical studies, 2nd edn. Berlin: Blackwell Science.
Teja-Isavadharm P., Nosten F., Kyle D.E. et al. (1996): Comparative bioavailability of oral, rectal and intramuscular artemether in healthy subjects: use of simultaneous measurement by high performance liquid chromatography and bioassay. Br. J. Clin. Pharmacol., 42, 599–604.
Van Agtmael M.A., Xiu-qing J., Koopmans R.P., Degen P., Royce C.M., Van Boxtel C.J. (1996): Multiple dose pharmacokinetics of artemether in Chinese patients treated for falciparum malaria. Abstracts of the VIth World Conference on Clinical Pharmacology and Therapeutics, Buenos Aires, Argentina, August 1996. Acta Physiologica Pharmacologica et Therapeutica Latinoamerica, p 244.
Kokwaro G.O. (1997): Bioavailability of artemether. Br. J. Clin. Pharmacol., 44, 303–305.
Karbwang J., Nabangchang K., Congpuong K., Molunto P. Thanavibul A. (1997): Pharmacokinetics and bioavailability of oral and intramuscular artemether. Eur. J. Clin. Pharmacol., 52, 307–310.
Oberle R.L., Amidon G.L. (1987): The influence of variable gastric emptying and intestinal transit rates on the plasma level curve of cimetidine; an explanation for the double peak phenomenon. J. Pharmacokinet. Biopharm., 15, 529–544.
Hammarlund M.M., Paalzow L.K., Odlind B. (1984): Pharmacokinetics of furosemide in man after intravenous and oral administration; application of moment analysis. Eur. J. Clin. Pharmacol., 26, 197–207.
Clements J.A., Heading R.C., Nimmo W.S., Prescott L.F. (1978): Kinetics of acetaminophen on absorption and gastric emptying in man. Clin. Pharmacol. Ther., 24, 420–431.
Lui C.Y., Oberle R., Fleisher D., Amidon G.L. (1986): A radiotelemetric method for evaluation of enteric coating performance: comparison of enteric-coated and plain aspirin tablets. J. Pharm. Sci., 75, 469–474.
Macheras P., Argyrakis P. (1997): Gastrointestinal drug absorption: is it time to consider heterogeneity as well as homogeneity? Pharm. Res., 14, 842–847.
Ngo Thu Hoa, Michoel A., Kinget R. (1996): Dissolution testing of artemisinin solid oral dosage forms. Int. J. Pharm., 138, 185–190.
Svensson U.S.H., Ashton M., Hai T.N. et al. (1996): Artemisinin and omeprazole pharmacokinetics in healthy male subjects after repeated co-administration; omeprazole as in vivo marker for CYP3A4 and 2C19. Abstracts of the XIVth International Congress for Tropical Medicine and Malaria, Nagasaki, Japan, November 1996. Abstr. P-01-76, p 341.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Van Agtmael, M.A., Van Der Graaf, C.A.A., Dien, T.K. et al. The contribution of the enzymes CYP2D6 and CYP2C19 in the demethylation of artemether in healthy subjects. Eur. J. Drug Metab. Pharmacokinet. 23, 429–436 (1998). https://doi.org/10.1007/BF03192305
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF03192305