Summary
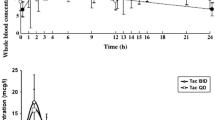
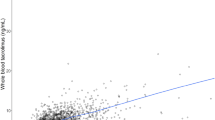
The pharmacokinetics of intravenous and oral tacrolimus was assessed in paediatric liver transplant patients at two centers in Europe. Sixteen patients, age 0.7 to 13 years, participated in the study; 12 patients were evaluable for intravenous pharmacokinetics, and 16 for oral. Intravenous tacrolimus was given as a continuous 24 h infusion (mean 0.037±0.013 mg/kg/day), and oral tacrolimus was given in 2 doses per day (mean 0.152±0.015 mg/kg). Whole blood samples for the intravenous pharmacokinetic profile were taken before initiation of the first infusion, 4, 8, 12 and 24 h post-infusion, and every 24 h thereafter until intravenous administration was discontinued. During the 12 h wash-out period between intravenous and oral administration, samples were taken every 3 h. Samples for the oral pharmacokinetic profile were taken immediately before the first oral dose and 0.5, 0.75, 1, 2, 2.5, 3, 4, 6, 8, 10 and 12 h post-administration. Non-compartmental procedures were used to characterise the pharmacokinetic parameters. Mean estimates for clearance and terminal half-life were 2.3±1.2 ml/min/kg and 11.5±3.8 h, respectively, following intravenous tacrolimus. The mean bioavailability of oral tacrolimus was 25±20%. A strong correlation was observed between AUC and trough whole blood levels of tacrolimus (r=0.90). The clearance was approximately 2-fold higher than that previously observed in adults; this could explain the higher dosage requirements in children.
Similar content being viewed by others
References
Tzakis A.G., Reyes J., Todo S. et al. (1993): Two-year experience with FK 506 in pediatric patients. Transplant. Proc., 25, 619–621.
Inomata Y., Tanaka K., Egawa H. et al. (1996): The evolution of immunosuppression with FK506 in pediatric living-related liver transplantation. Transplantation, 61, 247–252.
Tzakis A.G., Reyes J., Todo S. et al. (1991): FK 506 versus cyclosporine in pediatric liver transplantation. Transplant. Proc., 23, 3010–3015.
McDiarmid S.V., Busuttil R.W., Ascher N.L. et al. (1995): FK506 (tacrolimus) compared with cyclosporine for primary immunosuppression after pediatric liver transplantation. Results from the US multicenter trial. Transplantation, 59, 530–536.
Lee C., Jusko W., Shaefer M. et al. (1993): Pharmacokinetics of tacrolimus FK506 in transplant patients. Clin. Pharmacol. Ther., 53, 181.
Undre N., Möller A., the FK 506 European Study Group. (1994): Pharmacokinetic interpretation of FK 506 levels in blood and in plasma during a European randomised study in primary liver transplantation patients. Transplant. Int., 7 (Suppl. 1), S15-S21
Kobayashi M., Tamura K., Katayama N. et al. (1991): FK 506 assay past and present characteristics of FK 506 ELISA. Transplant. Proc., 3, 2725–2729.
Heinzel G., Woloszczak R., Thomann P. et al. (1993): TopFit: 2.0; pharmacokinetic and pharmacodynamic data analysis system for the PC. Berlin: Gustav Fischer. ISBN 3-437-11486-7.
Jain A.B., Fung J.J., Tzakis A.G. et al. (1991): Comparative study of cyclosporine and FK 506 dosage requirements in adult and pediatric orthotopic liver transplant patients. Transplant. Proc., 23, 2763–2766.
McDiarmid S.V., Colonna J.O., Shaked A. et al. (1993): Differences in oral FK506 dose requirements between adult and pediatric liver transplant patients. Transplantation, 55, 1328–1332.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Wallemacq, P.E., Furlan, V., Möller, A. et al. Pharmacokinetics of tacrolimus (FK506) in paediatric liver transplant recipients. Eur. J. Drug Metab. Pharmacokinet. 23, 367–370 (1998). https://doi.org/10.1007/BF03192295
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF03192295