Summary
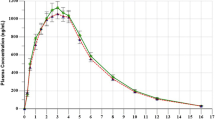
The aim of the present study was to investigate the relative bioavailability and bioequivalence of a new tablet formulation of metformin hydrochloride with reference to a standard product in healthy Chinese adult male volunteers. Two randomized, comparative, two-way crossover studies were therefore conducted. In study 1, which was a single-dose study, 20 subjects received 1000 mg metformin hydrochloride lest product extended-release (MXR) tablets followed by the same amount of metformin hydrochloride reference product immediate-release (MIR) tablets with a 7-day washout period between the two doses. In study 2, which was a multiple-dose study, 22 subjects received MXR 1000 mg/d for 9 consecutive days followed by MIR 1000 mg/d with a 14-day washout period between the doses of the test and reference product. The serum metformin concentrations were monitored using a selective and sensitive high-performance liquid chromatography (HPLC) method with ultraviolet (UV) detection. The pharmacokinetic parameters were calculated using a 3P97 program. Analysis of variance (ANOVA) of the half-life of the absorption phase (t1/2ka), the half-life of the elimination phase (t1/2kc) and the mean retention time (MRT) and the Wilcoxon Signed Rank test of Tmax for the two preparations were significantly different. A significant difference was found in the ANOVA for Cmax in the single-dose study, while this was not the case in the multiple-dose study. Two one-sided t-tests showed that there were no significant differences in the area under the concentration-time curve (AUC) values between the two formulations. The present study indicates that the test preparation was bioequivalent to the reference preparation when both MXR and MIR were investigated in healthy Chinese adult male volunteers. And on the basis of the mean AUC0−t, AUC0−∞ and AUCss, the relative bioavailability of the MXR was found to be 107.80%, 111.89% and 110.61% respectively compared with MIR.
Similar content being viewed by others
References
Scheen A.J., Lefebvre P.J. (1998): Oral antidiabetic agents. A guide to selection. Drugs, 55, 225–236.
DeFronzo R.A., Goodman A.M., the Multicenter Metformin Study Group. (1995): Efficacy of metformin in patients with non-insulin-dependent diabetes mellitus. The Multicenter Metformin Study Group. N. Engl. J. Med., 333, 541–549.
Garber A.J., Duncan T.G., Goodman A.M., Mills D.J., Rohlf J.L. (1997): Efficacy of metformin in type II diabetes: results of a double-blind, placebo-controlled, dose-response trial. Am. J. Med., 103, 491–497.
Reaven G.M., Johnston P., Hollenbeck C.B., Skowronski R., Zhang J.C., Goldfine I.D., Chen Y.D. (1992): Combined metformin- sulfonylurea treatment of patients with noninsulin-dependent diabetes in fair to poor glycémie control. J. Clin. Endocrinol. Metab., 74, 1020–1026.
Campbell I.W. (1985): Metformin and the sulphonylureas: the comparative risk. Horm. Metab. Res. (Suppl). 15, 105–111.
Stumvoll M., Nurjhan N., Peniello G., Dailey G., Gench J.E. (1995): Metabolic effects of metformin in non-insulin-dependent diabetes mellitus. N. Engl. J. Med., 333, 550–554.
Knowler W.C., Barrett-Connor E., Fowler S.E., Hamman R.F., Lachin J.M., Walker E.A., Nathan D.M.; Diabetes Prevention Program Research Group. (2002): Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N. Engl. J. Med., 346, 393–403.
Turner R., Cull C., Holman R. (1996): United Kingdom Prospective Diabetes Study (UKPDS) 17: a 9-year update of a randomized, controlled trial on the effect of improved metabolic control on complications in non-insulin-dependent diabetes mellitus. Ann. Intern. Med., 124 (1 pt 2), 136–145.
Marchetti P., Navalesi R. (1989): Pharmacokinetic-pharmacodynamic relationships of oral hypoglycaemic agents. An update. Clin. Pharmacokinet., 16, 100–128.
Bailey C.J., Turner R.C. (1996): Metformin. New Engl. J. Med., 334, 574–579.
Fujioka K., Pans M., Joyal S. (2003): Glycemie control in patients with type 2 diabetes mellitus switched from twice-daily immediate-release metformin to a once-daily extended-release formulation. Clin. Ther., 25, 515–529.
Hebden J.M., Gilchrist P.J., Blackshaw E.,Frier M.E., Perkins A.C., Wilson CG., Spiller R.C. (1999): Night-time quiescence and morning activation in the human colon: effect on transit of dispersed and large single unit formulations. Eur. J. Gastroenterol. Hepatol., 11, 1379–1385.
Huupponen R., Ojala-Karlsson P., Rouni J. Koulu M. (1992): Determination of metformin in plasma by high-performance liquid chromatography. J. Chromatogr., 583, 270–273.
Caille G., Laçasse Y., Raymond M., Landnault H., Perrotta M., Picirilh G., Thiffault J., Spenard J. (1993): Bioavailability of metformin in tablet form using a new high pressure liquid chromatography assay method. Biopharm. Drue Dispos., 14, 257–263.
Gusler G., Gorsline J., Levy G, Zhang S.Z., Weston I.E., Naret D., Berner B. (2001): Pharmacokinetics of metformin gastricretentive tablets in healthy volunteers. J. Clin. Pharmacol., 41, 655–661.
Cullen E., Liao J., Lukacsko P., Niecestro R., Friedhoff L. (2004): Pharmacokinetics and dose proportionality of extended-release metformin following administration of 1000, 1500, 2000 and 2500 mg in healthy volunteers. Biopharm. Drug. Dispos., 25, 261–263.
Depomed Corp. (2004): Metformin extended release — DepoMed: metformin, metformin gastric retention, metformin GR. Drugs RD, 5, 231–233.
Chow S.C., Liu J.P. (1992): Design and analysis of bioavailability and bioequivalence studies. New York: Marcel Dekker. Inc.
FDA. (1992): FDA Guidelines. Rockville, MD: Bioequivalence Food and Drug Administration, Division of Bioequivalence, Office of Generic Drugs, 1 July.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Li, J., Jin, Y., Wang, TY. et al. Relative bioavailability and bioequivalence of metforphin hydrochloride extended-released and immediate-released tablets in healthy Chinese volunteers. Eur. J. Drug Metabol. Pharmacokinet. 32, 21–28 (2007). https://doi.org/10.1007/BF03190986
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF03190986