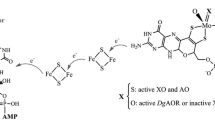
Summary
Both aldehyde oxidase and xanthine oxidase catalyze the oxidation of a wide range of N-heterocycles and aldehydes. These enzymes are important in the oxidation of N-heterocyclic xenobiotics, whereas their role in the oxidation of xenobiotic aldehydes is usually ignored. p ]The present investigation describes the interaction of methyl- and nitrosubstituted benzaldehydes, in theortho-,meta- andpara-positions, with guinea pig liver aldehyde oxidase and bovine milk xanthine oxidase.
The kinetic constants showed that most substituted benzaldehydes are excellent substrates of aldehyde oxidase with lower affinities for xanthine oxidase. Low Km values for aldehyde oxidase were observed with most benzaldehydes tested, with 3-nitrobenzaldehyde having the lowest Km value and 3-methylbenzaldehyde being the best substrate in terms of substrate efficiency (Ks). Additionally, low Km values for xanthine oxidase were found with most benzaldehydes tested. However, all benzaldehydes also had low Vmax values, which made them poor substrates of xanthine oxidase.
It is therefore possible that aldehyde oxidase may be critical in the oxidation of xenobiotic and endobiotic derived aldehydes and its role in such reactions should not be ignored.
Similar content being viewed by others
References
Feron V.J., Til H.P., de Vrijer F., Woutersen R.A., Cassée F.R., van Bladeren P.J. (1991): Aldehydes: occurrence, carcinogenic potential, mechanism of action and risk assessment. Mutat. Res. 259, 363–385.
Lindahl R. (1992): Aldehyde dehydrogenases and their role in carcinogenesis. Crit. Rev. Biochem. Mol. Biol. 27, 283–335.
Nursten H.E., Williams A.A. (1967): Fruit aromas: a survey of components identified. Chem. Ind. 12, 486–497.
Ma T.H., Harris M.M. (1988): Review of the genotoxicity of formaldehyde. Mutat. Res., 196, 37–59.
Dellarco V.L. (1988): A mutagenicity assessment of acetaldehyde. Mutat. Res. 195, 1–20.
Goodall McC, Alton H. (1968): Metabolism of 3-hydroxytyramine (dopamine) in human subjects. Biochem. Pharmacol. 17, 905–914.
Bond P.A. (1967): Metabolism of propranolol (‘Inderal’), a potent, specific beta-adrenergic receptor blocking agent. Nature 213, 721.
Washio K., Makaya O., Sasaki H., Nishida K., Nakamura J., Shibasaki J. (1993): A new aspect of tolbutamide metabolism in the rabbit: the role of l-butyl-3-(p-formylphenyl) sulphonylurea. J. Pharm. Pharmacol. 45, 231–233.
Panoutsopoulos G.I. (2005): Metabolism of homovanillamine to homovanillic acid in guinea pig liver slices. Cell. Physiol. Biochem. 15, 225–232.
Panoutsopoulos G.I., Kouretas D., Gounaris E.G., Beedham C. (20O4): Enzymatic oxidation of 2-phenylethylamine to phenylacetic acid and 2-phenylethanol with special reference to the metabolism of its intermediate phenylacetaldehyde. Basic Clin. Pharmacol. Toxicol. 95, 273–279.
Beedham C, Critchley D.J., Ranee D.J. (1995): Substrate specificity of human liver aldehyde oxidase toward substituted quinazolines and phthalazines: a comparison with hepatic enzyme from guinea pig, rabbit, and baboon. Arch. Biochem. Biophys. 319, 481–490.
Panoutsopoulos G.I., Beedham C. (2004): Enzymatic oxidation of phthalazine with guinea pig liver aldehyde oxidase and liver slices: inhibition by isovanillin. Acta Biochim. Pol. 51, 943–951.
Beedham C. (1987): Molybdenum hydroxylases: Biological distribution and substrate-inhibitor specificity. In: Ellis G.P., West G.B. (eds). Progress in Medicinal Chemistry. Elsevier, Amsterdam, Vol. 24, pp. 85–127.
Panoutsopoulos G.I., Beedham C. (2004) Kinetics and specificity of guinea pig liver aldehyde oxidase and bovine milk xanthine oxidase towards substituted benzaldehydes. Acta Biochim. Pol. 51, 649–663.
Krenitsky T.A., Neil S.M., Elion G.B., Hitchings G.H. (1972): A comparison of the specificities of xanthine oxidase and aldehyde oxidase. Arch. Biochem. Biophys. 150, 585–599.
Panoutsopoulos G.I., Kouretas D., Beedham C. (2004): Contribution of aldehyde oxidase, xanthine oxidase and aldehyde dehydrogenase on the oxidation of aromatic aldehydes. Chem. Res. Toxicol. 17, 1368–1376.
Morpeth F.F. (1983): Studies on the specificity toward aldehyde substrates and steady-state kinetics of xanthine oxidase. Biochim. Biophys. Acta 744, 328–334.
Sasaki K., Hosoya R., Wang Y.M., Raulston G.L. (1983): Formation and disposition of 7-hydroxymefhotrexate in rabbits. Biochem. Pharmacol. 32, 503–507.
Beedham C, Bruce S.E., Ranee D.J. (1987): Tissue distribution of the molubdenum hydroxylases, aldehyde oxidase and xanthine oxidase, in male and female guinea pigs. Eur. J. Drug Metab. Pharmacokinet. 12, 303–306.
Bruder G., Heid H., Jarasch E.D., Keenan T.W., Mather I.H. (1982): Characteristics of membrane-bound and soluble forms of xanthine oxidase from milk and endothelial cells of capillaries. Biochim. Biophys. Acta 701, 357–369.
Beedham C, Bruce S.E., Critchley D.J., Al-Tayib Y., Ranee D.J. (1987): Species variation in hepatic aldehyde oxidase activity. Eur. J. Drug Metab. Pharmacokinet. 12, 307–310.
Krenitsky T.A., Spector T., Hall W.W. (1986): Xanthine oxidase from human liver: purification and characterization. Arch. Biochem. Biophys. 247, 108–119.
Greek Presidential Decree No 160/1991 Protection of animals used for experimental and other scientific purposes in accordance with EU Directive 86/609/EEC of the Council. Governmental Gazette No 64.
Bradford M.M. (1976): A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72, 248–254.
Beedham C, Bruce S.E., Critchley D.J., Ranee D.J. (1990): 1-Substituted phthalazines as probes of the substrate-binding site of mammalian molybdenum hydroxylases. Biochem. Pharmacol. 39, 1213–1221.
Critchley D.J., Ranee D.J., Beedham C. (1992): Subcellular localisation of guinea pig hepatic molybdenum hydroxylases. Biochem. Biophys. Res. Commun. 185, 54–59.
Rajagopalan K.V., Handler P. (1964): Hepatic aldehyde oxidase: the substrate-binding site. J. Biol. Chem. 239, 2027–2035.
Wilkof CA., Korus R.A., Crawford D.L., Pometto III A.L. (1984): Enzymic oxidation of aromatic aldehydes. Biotechnol. Bioeng. Symp. (Symp.Biotechnol.-Fuels Chem. 6th), 14, 419–423.
Coughlan M.P. (1980) In: Coughlan M.P. (ed). Molybdenum and molybdenum-containing enzymes. Pergamon Press, Oxford.
Taylor S.M., Stubley-Beedham C, Stell J.G.P. ( 1984): Simultaneous formation of 2- and4-quinolones from quinolinium cations catalysed by aldehyde oxidase. Biochem. J. 220,67–74.
Spector T., Hall W.W., Krenitsky T.A. (1986): Human and bovine xanthine oxidases: inhibition studies with oxipurinol. Biochem. Pharmacol. 35, 3109–3114.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Veskoukis, A.S., Kouretas, D. & Panoutsopoulos, G.I. Substrate specificity of guinea pig liver aldehyde oxidase and bovine milk xanthine oxidase for methyl- and nitrobenzaldehydes. European Journal of Drug Metabolism and Pharmacokinetics 31, 11–16 (2006). https://doi.org/10.1007/BF03190636
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF03190636