Summary
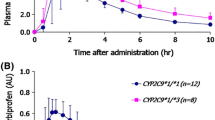
Diclofenac has been used for the evaluation of CYP2C9 activityin vitro as well asin vivo with varying results. The present study was aimed at evaluating the reproducibility of the urinary diclofenac/4′-OH diclofenac ratio among differentCYP2C9 genotypes in healthy volunteers. The study ofCYP2C9 genotypes in the family of aCYP2C9*3/*3 subject is also reported. The urinary diclofenac/4′-OH diclofenac ratio was determined on two occasions within a period of 9–12 months, and was found to be correlated (r=0.83,p<0.05). The mean (±SD) of diclofenac/4′-OH diclofenac ratio was 1.5 times higher among subjects carryingCYP2C9*3 allele (CYP2C9*1/*3 andCYP2C9*2/*3 genotypes) (0.91±0.28), compared toCYP2C9*1/*1 subjects (0.60±0.11). The results show that the urinary diclofenac/4′-OH diclofenac ratio might be used to study CYP2C9 in humans. The data agree with previous studies showing that theCYP2C9*3 allelic variant seems to cause a decreased CYP2C9 hydroxylation capacity.
Similar content being viewed by others
References
Xie H.G., Prasad H.C., Kim R.B., Stein C.M. (2002):CYP2C9 allelic variants: ethnic distribution and functional significance. Adv. Drug. Deliv. Rev., 54, 1257–1270.
Miners J.O., Birkett D.J. (1998): Cytochrome P4502C9: an enzyme of major importance in human drug metabolism. Br. J. Clin. Pharmacol., 45, 525–538.
Lee C.R., Goldstein J.A., Pieper J. (2002): Cytochrome P4502C9 polymorphisms: a comprehensive review of the in vitro and human data. Pharmacogenetics, 12, 251–263.
Shimamoto J., Ieiri I., Urae A., et al. (2000): Lack of differences in diclofenac (a substrate for CYP2C9) pharmacokinetics in healthy volunteers with respect to the singleCYP2C9*3 allele. Eur. J. Clin. Pharmacol., 56, 65–68.
Morin S., Loriot M.A., Poirier J.M., et al. (2001): Is diclofenac a valuable CYP2C9 probe in humans? Eur. J. Clin. Pharmacol., 56, 793–797.
Yasar Ü., Eliasson E., Forslund-Bergengren C., et al. (2001): The role ofCYP2C9 genotype in the metabolism of diclofenac in vivo and in vitro. Eur. J. Clin. Pharmacol., 57, 729-Xie 735.
Dorado P., Berecz R., Cáceres M.C., LLerena A. (2003): Analysis of diclofenac and its metabolites by high-performance liquid chromatography: Relevance ofCYP2C9 genotypes in diclofenac urinary metabolic ratios. J. Chromatogr B., 789, 437–442.
Dorado P., Berecz R., Norberto M.J., Yasar Ü., Dahl M.-L., LLerena A. (2003):CYP2C9 genotypes and diclofenac metabolism in Spanish healthy volunteers. Eur. J. Clin. Pharmacol. (in press).
Yasar Ü., Forslund-Bergengren C., Tybring G., et al. (2002): Pharmacokinetics of losartan and its metabolite E-3174 in relation to theCYP2C9 genotype. Clin. Pharmacol. Ther., 71, 89–98.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Dorado, P., Berecz, R., Cáceres, M.C. et al. Reproducibility over time of the urinary diclofenac/4′-OH diclofenac ratio among differentCYP2C9 genotypes. Eur. J. Drug Metab. Pharmacokinet. 28, 213–215 (2003). https://doi.org/10.1007/BF03190487
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF03190487