Summary
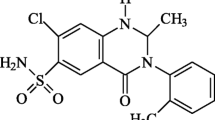
In a randomized 2-way cross-over study with eighteen healthy male volunteers, two moxonidine preparations (tablets, treatment A vs. intravenous solution, treatment B) were tested to investigate absolute bioavailability and pharmacokinetics of moxonidine. The preparations were administered as single doses of 0.2 mg; prior to and up to 24 h after administration blood samples were collected and the plasma moxonidine concentrations determined. Urine samples were collected prior to and at scheduled intervals up to 24 h after administration for the determination of unchanged moxonidine. Moxonidine plasma and urine concentrations were determined by a validated gas chromatographic/mass spectrometric method with negative ion chemical ionization. The mean areas under the plasma concentration/time curves were calculated as [mean ± standard deviation] 3438 ± 962 pg.h/ml (AUC(0→Tlast)) and 3674 ±1009 pg.h/ml (AUC(0→∞)) for treatment A; 3855 ± 1157 pg.h/ml (AUC(0→Tlast)) and 4198 ± 1205 pg.h/ml (AUC(0→∞)) for treatment B. Mean peak plasma concentrations of 1495 ± 646 pg/ml were attained at 0.56 ± 0.28 h after oral treatment, mean peak plasma concentrations after intravenous treatment reached 3965 ± 1342 pg/ml at 0.17 ± 0.01 h (= coinciding with end of infusion). The mean terminal half-lives of moxonidine were derived as 1.98 h after administration of the tablet and as 2.18 h after infusion. The amounts of moxonidine excreted in urine during the 24 h following administration (Ae(24h)) in absolute figures and as percentage of thedose administered were 102 ± 26 μg or 51 ± 13% for the tablet and 122 ± 33 μg or 61 ± 16% for the infusion.
The relative bioavailability of oral moxonidine (assuming the availability of the i.v. solution to be 100%) was calculated to be 89% or F=0.89 (AUC(0→Tlast)) and 88% or F=0.88 (AUC(0→∞)). Taking these results into account, the relative amount of moxonidine excreted in urine of thedose absorbed comes up to approximately 58% for the tablet, which is comparable to an excretion of 61% of the dose administered (= absorbed) after i.v. administration. With the self-evident exception of Cmax, statistical testing of pharmacokinetic parameters revealed no significant differences between treatments.
Similar content being viewed by others
References
Armah B.I. (1987): Contributions of presynaptic α2-adrenoceptor stimulation to the antihypertensive action of moxonidine. J. Cardiovasc. Pharmacol., 10 (Suppl. 4), 81–83.
Armah B.I., Hofferber E., Stenzel W. (1988): General pharmacology of the novel centrally acting antihypertensive agent moxonidine. Arzneimittelforsch., 38 (II), 10, 1426–1434.
Bergerhausen J. (1985): Moxonidine (BE S89S), a novel full agonist at human platelet alpha-2-adrenoceptors. Naunyn-Schmiedeberg Arch. Pharmacol., 329, R80.
Kukovetz W.R., Diembeck W. (1986): Pharmacokinetic study after intravenous and oral administration of11C-moxonidine to humans. Unpublished observations.
Trenk D., Wagner F., Jähnchen E., Planitz V. (1987): Pharmacokinetics of moxonidine after single and repeated daily doses in healthy volunteers. J. Clin. Pharmacol., 27, 988–993.
Frisk-Holmberg M., Planitz V. (1987): A selective (α2-adrenoceptor agonist in arterial essential hypertension. Curr. Then Res., 42,138–146.
Investigators Brochure MOXONIDINE (1988): Hannover, Kali-Chemie AG.
Mitrovic V., Patyna W.D., Husseini H., Volz M., Schlepper M. (1989): Hemodynamic and neurohumoral effects of moxonidine in hypertensive patients after an oral single dose of 0.4 mg. Cardiovasc. Drugs Ther., 3 (Suppl. 2), 610.
Häring N., Salama Z.B. (1989): Determination of moxonidine in human plasma by gas chromatography/mass spectrometry with negative ion chemical ionization. Unpublished L.A.B. GmbH & Co Validation Report
Schuirman D.L. (1987): A comparison of the two one-sided tests procedure and the power approach for assessing the equivalence of average bioavailability. J. Pharmacokmet Biopharm., 15,657–680.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Theodor, R., Weimann, H.J., Weber, W. et al. Absolute bioavailability of moxonidine. European Journal of Drug Metabolism and Pharmacokinetics 16, 153–159 (1991). https://doi.org/10.1007/BF03189952
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF03189952