Summary
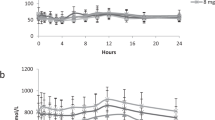
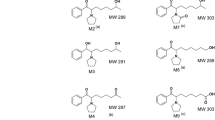
The metabolic fate of the anti-Parkinsonian drug budipine was studied in rats after oral administration. The presence of an aromatic hydroxylation product, metabolite Mi, and its O-sulphate conjugate was confirmed. Three new minor metabolites, budipine N-oxide, metabolite M1 N-oxide and a secondary metabolite derived from M1 via hydroxylation of a methyl of thetert-butyl group, were isolated and identified in rat urine. The presence of a metabolite M1-glucuronic acid conjugate, was also established through different enzymatic treatments of the rat urine.
Similar content being viewed by others
References
Iizuka J., Fischer R. (1986): Beeinflussung des Parkinson-tremors durch Budipin. Nervenarzt, 37, 184–186.
Xinde Wang (1985): Observation on the therapeutic effect of budipine in Parkinson’s disease. In: Gerstenbrand F., Poewe W., Stern G. Eds. Clinical Experiences with Budipine. Berlin, Heidelberg, New York, Springer, pp. 158–162.
Jellinger K., Bliesath H. (1987): Adjuvant treatment of Parkinson’s disease with budipine: a double-blind trial versus placebo. J. Neurol. 234, 280–282.
Offermeier J., Van Rooyen J.M. (1985): The pharmacodynamics of budipine on central neurotransmitter systems. In: Gerstenbrand F., Poewe W., Stern G. Eds. Clinical Experiences with Budipine. Berlin Heidelberg, New York, Springer, pp. 93–106.
Zech K., Sturm E., Ludwig G. (1985): Pharmacokinetics and metabolism of budipine in animals and humans. In: Gerstenbrand F., Poewe W., Stern G. Eds. Clinical Experiences with Budipine. Berlin Heidelberg, New York, Springer, pp. 113–121.
Schaefer H., Hackmack G., Eistetter K., Kruger V., Menge H.G., Klose J. (1984): Synthese, physicalisch-chemische eigenschaften und orientirende pharmacologische Untersuchungen von budipin und verwandten 4,4-diphenylpiperidinen. Arzneimittelforsch., 34, 233–240.
Becker H.G.O., Fanghaenel E. (1964): Preparation of Mannich bases with reversibly blocked nitrogen atom. J. Prep. Chem., 26, 58–66.
Caputo O., Grosa G., Balliano G., Rocco F., Biglino G. (1988): In vitro metabolism of 2-(5-ethylpyridin-2-yl)benzimidazole. Eur. Drug Metab. Pharmacokinet., 13, 47–51.
Kamm J.J., Szuna A., Kuntzman R. (1972): Studies on unusual N-dealkylation reaction. I. In vivo and in vitro N-dealkylation of N-tert-butylchlorcyclizine to norchlorcyclizine by the rat. Pharmacol. Exp. Ther., 182, 507–514.
Leitch R.E. (1971): Precise quantitative analysis with stable high-speed liquid-liquid chromatography column. J. Chromatogr., 9, 531–535.
Author information
Authors and Affiliations
Additional information
This work was supported by M. U. R. S. T.
Rights and permissions
About this article
Cite this article
Caputo, O., Grosa, G., Ceruti, M. et al. The metabolic fate of the anti-Parkinsonian drug budipine in rats. European Journal of Drug Metabolism and Pharmacokinetics 16, 113–118 (1991). https://doi.org/10.1007/BF03189947
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF03189947