Summary
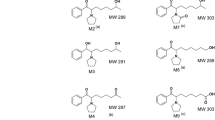
A total of 16 metabolites of bromocriptine could be isolated from rat bile and incubates of rat liver cell preparations using [6-methyl-14C]bromocriptine as substrate. Separation and purification was achieved by reversed-phase liquid chromatography and preparative thin-layer chromatography in conjunction with radioactivity monitoring. Structure elucidation was based on spectroscopic data (UV, IR, NMR, EI- and FD-MS) and the results of amino acid analysis after acid hydrolysis.
Based on the identified metabolites four principal transformation processes could be described :
-
— Hydrolytic cleavage of the amide bridge leading to the formation of 2-bromolysergic acid amide (3) and 2-bromolysergic acid (7).
-
— epimerization at position 8 of the bromolysergic acid moiety to the iso-derivatives (isobromocriptine, 2-bromo-isolysergic acid (6), its amide (1), etc.)
-
— regiospecific oxidation at position 8′ in the proline fragment generating stereoisomeric 8′-hydroxy-bromocriptines (21–24)
-
— further oxidation of the 8′-hydroxylated derivatives by either the introduction of a second hydroxy group at position 9′ to give dihydroxylated derivatives (detected as conjugates with glucuronic acid: metabolites29, 30 and31), or the opening of the proline ring under formation of the metabolites4 and5 containing glutamic acid instead of proline (7′, 8′-seco-8′-carboxy-bromocriptines).
It is suggested that the primary and principal metabolic attack occurs at the proline fragment of the drug. In contrast to the biotransformation of ergoline compounds, none of the bromocriptine metabolites detected showed oxidative transformations in the lysergic acid half of the molecule.
Similar content being viewed by others
References
Thorner, M.O., Flückiger, E., Calne, D.B. (1980): Bromocriptine, a clinical and pharmacological review. Raven Press, New York.
Eckert, H., Kiechel, J.R., Rosenthaler, J. Schmidt, R., Schreier, E. (1978): Biopharmaceutical aspects. Analytical methods, pharmacokinetics, metabolism and bioavailability. In: Berde, B., Schild, H.O. (eds.); Ergot alkaloids and related compounds. Handbook Exp. Pharmacol.49, 719–813; Berlin, Heidelberg, New York: Springer.
Meier, J., Schreier, E. (1976): Human plasma levels of some anti-migraine drugs. Headache16, 96–104.
Voges, R., Schreier, E.: Labelling of bromocriptine with carbon-14 in the 6-methyl group. Sandoz Ltd. Basle, internal reports 1974 and 1979, unpublished.
Seglen, O. (1976): Preparation of isolated rat liver cells. Methods Cell Biol.13, 29–83.
Paul, J. (1975): Cell and Tissue Culture 5th ed., p. 368. Edinburgh, London and New York: Churchill Livingstone.
Maurer, G. (1977): Isolement et structure de métabolites biliaires de la dihydro-β-ergokryptine chez le rat. Thèse de docteur d’état es-sciences présentée devant l’Institut National des Sciences Appliquées de Lyon et l’Université Claude Bernard Lyon I. No d’ordre IDE 77014.
Markey, S.P., Colburn, R.W., Kopin, I.J. Aamodt, R.L. (1979): Distribution and excretion in the rat and monkey of [82Br]bromocriptine. J. Pharmacol. Exp. Ther.211, 31–35.
Wong, S.H., Spencer, R.P., Hosain, P. (1978): Distribution and fate of 2-Br-82-alpha-ergokryptine and H-3-dihydroergokryptine in female rabbits. Fed. Proc.37, 741, Abstract No. 2773.
Stetten, M.R. (1955): Metabolic relationship between glutamic acid, proline, hydroxyproline and ornithine. In: A symposium on amino acid metabolism. Mc Elroy, W.D. and Glass, H.B. (eds). Baltimore: The John Hopkins Press.
Parke, D.V. (1968): The biochemistry of foreign compounds. London: Pergamon Press.
Ho, B., Castagnoli, N. (1980): Trapping of metabolically generated electrophilic species with cyanide ion. Metabolism of 1-benzylpyrrolidine. J. Med. Chem.23, 133–139.
Testa, B., Jenner, P. (1976): Drug metabolism. Chemical and biochemical aspects, pp. 83–97. New York: Marcel Dekker.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Maurer, G., Schreier, E., Delaborde, S. et al. Fate and disposition of bromocriptine in animals and man. I: Structure elucidation of the metabolites. European Journal of Drug Metabolism and Pharmacokinetics 7, 281–292 (1982). https://doi.org/10.1007/BF03189631
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF03189631