Abstract
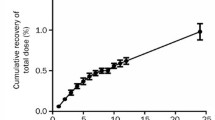
Concentration-time profiles of125I-labeled recombinant human interleukin-3 (1251rhIL-3) were determined by reverse phase high performance liquid chromatography (RHPLC) after intravenous and subcutaneous administration of the drug in 16 rhesus monkeys. The initial and terminalT 1/2 in plasma after intravenous of 30 μg/kg were (0.15 ± 0.13) and (2.21 ± 0.59) h, respectively. Terminal half-lives after 30, 90 and 180 μg/kg subcutaneous (s. c.) injections were 2. 0–3. 8 h. Area under concentration-time curves (AUC) following s. c. were roughly increased with dose, while CLs were similar among different dosages. The absorption rates were dependent on concentration at injected site. Bioavailability was about 0.7 after s. c. Rapid biodegradation was found in plasma. Distribution profiles of total radioactivity were as follows: the highest level was found in urinary system; levels in bile-enteric system, lymph nodes, bone marrow and spleen were near to that in plasma, and level in brain was the lowest. The RH-PLC analysis revealed that kidney was one of the major organs for biodegradation.
Similar content being viewed by others
References
Bot, F.J., Dorssers, L.C.J., Wagemaker, G.el a1., Stimulating spectrum of human recombinant multi-CSF(IL-3) on human marrow precursors: importance of accessory cells,Blood, 1988. 67: 1609.
Ganser, A., Lindemann, A., Seipelt, G.et al., Effects of recombinant human interleukin-3 in patients with normal hernatopoiesis and in patients with bone marrow failure,Blond, 1990, 76: 666.
Yang, Y.C., Ciarletta, A. B., Temple, P.A.et al., Human IL-3(multi CSF): Identification by expression cloning of a novel hematopoietic growth factor related to murine IL-3.Cell. 1986. 47: 3.
Tang, Z.M., Liu, X.W., Xu, L.P.et al., Pharmacokinetics and tissue distribution of human recombinant interleukin-2 in mice,Acta Pharmacologica Sinica, 1994, 15: 51.
van Gils, F. C., Westerrnan, Y., van den Bos, C.et al., Pharmacokinetic basis for optimal hematopoietic effectiveness of homologous IL-3 administered to rhesus monkeys,Leukemia. 1993, 7: 1602.
Welling, P.G., Balant, L.P., eds.,Pharrnacokinetlcs of Drugs, Berlin Heidelberg: Springer-Verlag, 1994, 83–98.
Tang, Z.M., Liu, X.W., Chai, B.X.et al., Methodology and experimental design in pharmacokinetics of proteins and peptides,Chinese J. Pharmacol. Toxicol. (in Chinese), 1996, 10: 161.
Biesma, B., Pokorny, R., Kovarik, J.M.et al., Pharrnacokinetics of recombinant human interleukin 3 administrated subcutaneously and by continuous intravenous infusion in patients after chemotherapy for ovarian cancer,Cancer Res., 1993, 53: 5915.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Tang, Z., Liu, X. & Tu, M. Pharmacokinetics of recombinant human interleukin-3 in rhesus monkeys. Sci. China Ser. C.-Life Sci. 40, 546–553 (1997). https://doi.org/10.1007/BF03183595
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF03183595