Abstract
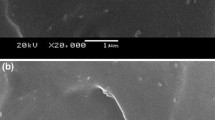
Spherical calcium carbonate composite is synthesized in the solution of amphiphilic block copolymer of polystyrene(PS) and poly(acrylic acid)(PAA). SEM and XRD measurements show that the diameter of the particulates decreases with the augment of the PS-b-PAA concentration, crystalline in the composite is calcite and its morphology as well as the structure is changed too. TG-DTA together with IR analysis is applied to investigating the thermal dynamic behavior of the composite. The results show that the composite is mainly composed of two phases, that is, the nano-crystalline calcium carbonate and the PS-b-PA-Ca composites. PS phase decomposes first with a large heat release at about 330 °C. However, the PAA chains have relatively high thermal stability, probably due to the structural Ca-O bond, and decomposes at above 400 °C. Matching opinions are used to explain the possible reasons for the regular as well as the particular characteristics of the composite corresponding to a certain copolymer concentration.
Similar content being viewed by others
References
Aizenberg, J., Tkachenko, A., Weiner, S. et al., Calcitic microlenses as part of the photoreceptor system in brittlestars, Nature, 2001, 412: 819–822.
Falini, G., Albeck, S., Weiner, S. et al., Control of aragonite or calcite polymorphism by mollusk shell macromolecules, Science, 1996, 271(5245): 67–69.
Smith, B. L., Schaffer, T. E., Viani, M. et al., Molecular mechanistic origin of the toughness of natural adhesives, fibres and composites, Nature, 1999, 399(6738): 761–763.
Heywood, B. R., Mann, S., Template-directed nucleation and growth of inorganic materials, Adv. Mater., 1994, 6: 9–20.
Aizenberg, J., Black, A. J., Whitesides, G. M., Control of crystal nucleation by patterned self-assembled monolayers, Nature, 1999, 398(6727): 495–498.
D’Souza, S. M., Alexander, C., Carr, S. W. et al., Directed nucleation of calcite at a crystal-imprinted polymer surface, Nature, 1999, 398(6725): 312–316.
Falini, G., Fermani, S., Gazzano, M. et al., Biomimetic crystallization of calcium carbonate polymorphs by means of collagenous matrices, Chem. Eur. J., 1997, 3(11): 1807–1814.
DeOliveira, D. B., Laursen, R. A., Control of calcite crystal morphology by a peptide designed to bind to a specific surface, J. Am. Chem. Soc., 1997, 119(44): 10627–10631.
Colfen, H., Qi, L., A systematic examination of the morphogenesis of calcium carbonate in the presence of a double-hydrophilic block copolymer, Chem. Eur. J., 2001, 7: 106–116.
Colfen, H., Antonietti, M., Crystal design of calcium carbonate microparticles using double-hydrophilic block copolymers, Langmuir, 1998, 14: 582–589.
Zhang, L. F., Eisenberg, A., Multiple morphologies of “crew-cut” aggregates of polystyrene-b-poly(acrylic acid) block copolymers, Science, 1995, 268: 1728–1731.
Moffitt, M., Mcmahon, L., Pessel, V. et al., Size control of nanoparticles in semiconductor-polymer composites. 2. Control via sizes of spherical ionic microdomains in styrene-based diblock ionomers, Chem. Mater., 1995, 7: 1185–1192.
Yue, L. H., Shui, M., Xu, Z. D. et al., The crystal structure of ultra fine CaCO3 and its thermal decomposition, Chemical Journal of Chinese University, 2000, 21(10): 1555–1559.
Mann, S., Heywood, B. R., Rajam, S. et al., Controlled crystallization of CaCO3 under steatic-acid monolayers, Nature, 1988, 334(6184): 692–695.
Amir, B., Dong, J. A., Anna, L. et al., Total alignment of calcite at acidic polydiacetylene films: cooperativity at the organic-inorganic interface, Science, 1995, 269: 515–518.
Lara, A. E., Andrew, D. H., At the interface of organic and inorganic chemistry: bioinspired sythesis of composite materials, Chem. Mater., 2001, 13: 3227–3235.
Yu, S. H., Colfen, H., Hartmann, J. et al., Biomimetic crystallization of calcium carbonate spherules with controlled surface structures and sizes by double-hydrophilic block copolymers, Adv. Funct. Mater., 2002, 12(8): 541–545.
Mann, S., Didymus, J. M., Sanderson, N. P. et al., Morphological influence of functionalized and non-functionalized α, ω-dicarboxylates on calcite crystallization, J. Chem. Soc. Faraday, 1990, 86(10): 1873–1880.
Zhang, J., Gonsalves, K. E., Synthesis of calcium carbonate-chitosan composites via biomimetic processing, J. Applied Polymer Science, 1995, 56: 687–695.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Yue, L., Jin, D. The synthesis of spherical calcium carbonate composite in amphiphilic PS-b-PAA solution and its thermal dynamic characteristic. Chin.Sci.Bull. 49, 235–239 (2004). https://doi.org/10.1007/BF03182804
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF03182804