Abstract
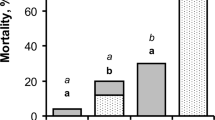
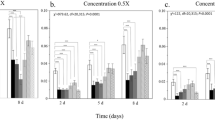
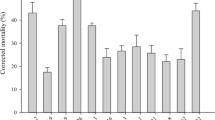
The giant Madagascar hissing-cockroach,Gromphadorhina portentosa, and its mite associate,Gromphadorholaelaps schaeferi, constitute an intimate commensalistic symbiosis. While the mite’s very survival is dependent by feeding on cockroach saliva and associated organic debris, the degree that the cockroach benefits from this association is unclear. We investigated the mite’s potential role at regulating surface fungi on the exoskeletons of this insect. Numbers of fungal isolates that resulted were compared between captive-bred cockroaches with and without mites. The mycoflora of both groups consisted of common molds (Alternaria sp.,Aspergillus sp.,Cladosporium sp.,Geotrichum sp.,Mucor sp.,Penicillium sp.,Rhizopus sp.,Trichoderma sp.). The presence of mites reduced the number of isolates by 1/2 in mature females, 1/3 in males, and 1/4 in sixth (final) instar nymphs. Fungus levels continued to drop when mite-free cockroaches were artificially supplemented with mites. A direct correlation was detected between mites and the reduction in the quantity of surface molds up to 20 mites per cockroach. The addition of more mites above 20 per cockroach, even 4x more, had a minimal, but still reducing, effect. Mites regulated all types of fungi, not just a select few taxa. We propose that mites reduce the mycoflora not because they consume fungi, but because mites and molds compete for the same resources in an ecological niche, saliva and organic debris that accumulate in between cockroach’s legs. Cockroaches reared in captivity do not apparently benefit by the removal of surface molds by mites, lending support for a commensalistic symbiosis. This cockroach species has been linked to severe allergic reactions in children, in part, because it harbors antagonistic molds. GivenG. schaeferi’s regulatory role at suppressing fungi, these mites could conceivably impose a small indirect, albeit beneficial role to humans by reducing the amount of fungal inoculum (conidia) that might otherwise be inhaled.
Similar content being viewed by others
References
Bamett, H.L. and Hunter, B.B. 1998.Illustrated Genera of Imperfect Fungi. 4th edition, APS Press, St. Paul, MN. 218 pp.
Dubus, J.C., Guerra M.T., and Bodios, A.e. 2001. Cockroach allergy and asthma.Allergy 56: 351–358.
Egan, M.E. and Moss. W.W. 1969. The life cycle and behavior of a cockroach mite.Proctolaelaps nauphoetae (Acari: Mesostigmata: Ascidae).Notulae Naturae 420: 1–9.
Egan. M.E., Barth, R.H.. and Hanson, F.E. 1975. Chemicallymediated host selection in a parasitic mite.Nature 257: 788–790.
Eggleston, P.E. and Arruda, L.K. 2001. Ecology and elimination of cockroaches and allergens in the home.Journal of Allergy and Clinical Immunology 107: S422-S429.
Fotedar, R. and Banerjee, U. 1992. Nosocomial fungal infectionsstudy of the possible role of cockroaches (Blattella germanica) as vectors.Acta Tropica 50: 339–343.
Gerdeman, B.S.. Klompen, J.S.H., and Yoder, LA. 1998. The larva ofGromphadorholaelaps schaeferi Till (Acari: Laelapidae), an associate of the Madagascar hissing-cockroach.Gromphadorhina portentosa (Schaum).International Journal of Acarology 24: 301–305.
Jennings, D.H. and Lysek, G. 1999.Fungal Biology: Understanding the Fungal Lifestyle. Scientific Publishers Limited. Oxford. United Kingdom. 166 pp.
Karg, W. 1991. New species of the genusAndrolaelaps Berlese (Mesostigmata: Laelapidae) from a cockroach in Madagascar.International Journal ofAcarology 17: 165–168.
Lemos, A.A., Lemos. JA, Prado, M.A., Pirnenta, F.e., Gir, L Silva, H.M., and Silva, M.R.R. 2006. Cockroaches as carriers of fungi of medical importance.Mycoses 49: 23–25.
Marx. D.H. 1969. The influence of ectotrophic mycorrhizal fungi on the resistance of pine roots to pathogenic infections. I. Antagonism of mycorrhizal fungi to root pathogenic fungi and soil bacteria.Phytopathology 59: 153–163.
Morgan, M.S., Arlian, L.G., Bernstein, lA., and Yoder, I.A. 2007. Allergenicity of the Madagascar hissing cockroach.Annals of Allergy, Asthma and Immunology 98: 258–261.
Nelson, M.C. and Fraser, J. 1980. Sound production in the cockroach,Gromphadorhina portentosa: Evidence for communication by hissing.Behavioral Ecology and Sociobiology 6: 305–314.
Roth, L.M. and Willis, E.R. 1960. The biotic assocrations of cockroaches.Smithsonian Miscellaneous Collections 141: 1–470.
Schaefer. C.W. and Peckham, D.B. 1968. Host preference studies on a mite infesting the cockroachGromphadorhina portentosa.Annals ofthe Entomological Society ofAmerica 61: 1475–1478.
Sokal, R.R. and Rohlf, F.J. 1995.Biometry: The Principles and Practice of Statistics in Biological Research. W.H. Freeman, San Francisco, CA. 887 pp.
Till. W.M. 1969. A new laelapine mite from the Madagascar hissing-cockroach.Gromphadorhina portentosa (Schaum).Acarologia 11: 515–523.
Yoder. J.A. 1996. The Madagascar hissing-cockroach mite (Gromphadorholaelaps schaeferi): First observation of its larva and ptyalophagy in the Acari.International Journal of Acarology 22: 141–148.
Yoder, J.A. 1997. Exterminator-mites (Acari: Dermanyssidae) on the giant Madagascar hissing-cockroach.International Journal ofAcarology 23: 233–236.
Yoder, J.A. and Barcelona, J.C. 1995. Food and water resources used by the Madagascan hissing-cockroach mite.Gromphadorholaelaps schaeferi.Experimental and Applied Acarology 19: 259–273.
Yoder, l.A. and Grojean, N.C. 1997. Group influence on water conservation in the giant Madagascar hissing-cockroach.Gromphadorhina portentosa. Physiological Entomology 22: 7982.
Yoder. J.A.. Glenn. B.D.. Benoit, I.B.. and Zettler. L.W. 2008. The giant Madagascar hissing-cockroach (Gromphadorhina portentosa) as a source of antagonistic moulds: concerns arising from its use in a public setting.Mycoses 51: 95–98.
Yoder, J.A., Mahmood, S.S., and Lalli, P.N. 2001. Semiochemical parsimony between the Madagascar hissing-cockroach mite and its host.International Journal of Acarology 27: 139–143.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yoder, J.A., Chambers, M.J., Condon, M.R. et al. Regulation of the external mycoflora of the giant Madagascar hissing-cockroach,gromphadorhina portentosa, by its mite associate,gromphadorholaelaps schaeferi, and its implications on human health. Symbiosis 47, 93–98 (2009). https://doi.org/10.1007/BF03182292
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF03182292