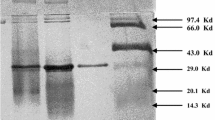
Abstract
Cyclodextrin glucanotransferase (CGTase, EC 2.4.1.19) fromBacillus circulans ATCC 21783 was purified by ultrafiltration and a consecutive starch adsorption. Total enzyme yield of 75.5% and purification factor of 13.7 were achieved. CGTase was most active at 65°C, possessed two clearly revealed pH-optima at 6.0 and 8.6 and retained from 75 to 100% of its initial activity in a wide range of pH, between 5.0 and 11.0. The cyclising activity was enhanced by 1 mM CaCl2 or 4 mM CoCl2. The enzyme was thermostable up to 70°C, and 64% of the original activity remained at 70°C after 30 min heat treatment. Up to 41% conversion into cyclodextrins was obtained from 40 g l−1 starch without using any additives. This CGTase produced two types of cyclodextrins, beta and gamma, in a ratio 73:27 after 4 h reaction time at 65°C. This feature of the enzyme could be of interest for industrial cyclodextrin production.
Similar content being viewed by others
References
Akimaru K., Yagi T., Yamamoto S. (1991). Purification and properties ofBacillus coagulans cyclomaltodextrin glucanotransferase. J. Ferm. Bioeng., 71: 322–328.
Boveto L.J., Bacher D.P., Villete J.R., Sicard P.J., Bouquelet S.J.L. (1992). Cyclomaltodextrin glucanotransferase fromBacillus circulans E 192. Purification and characterization of the enzyme. Biotechnol. Appl. Biochem., 15: 48–58.
Bradford M.M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem., 72: 248–254.
Cao X., Jin Z., Wang X., Chen F. (2005). A novel cyclodextrin glycosyltransferase from an alkalophilicBacillus species: purification and characterization. Food Res. Int. 38: 309–314.
Cepeda A., Franco C.M., Fente C.A., Vazquez B.I., Rodriguez J.L., Prognon P., Mahuzier G. (1996). Postcolumn extraction of aflatoxins using cyclodextrins in liquid chromatography for food analysis. J. Chromatography A, 721 (1): 69–74.
Chung H.J., Yoon S.H., Lee M.J., Kim M.J., Kweon K.S., Lee I.W., Kim J.W., Oh B.H., Lee H.S., Spiridonova V.A., Park K.H. (1998). Characterization of a thermostable cyclodextrin glucanotransferase isolated fromBacillus stearothermophilus ET1. J. Agr. Food Chem., 46: 952–959.
Fente C.A., Ordaz J.J., Vazquez B.I., Franco C.M., Cepeda A. (2001). New additive for culture media for rapid identification of aflatoxin-producingAspergillus strains. Appl. Environ. Microbiol., 67 (10): 4858–4862.
Fujita Y., Tsubouchi H., Inagi Y., Tomita K., Ozaki A., Nakamura K. (1990). Purification and properties of cyclodextrin glycosyltransferase fromBacillus sp. AL-6. J. Ferment. Bioeng., 70: 150–154.
Fujiwara S., Kakihara H., Sakaguchi K., Imanaka T. (1992). Analysis of mutation in cyclodextrin glucanotransferase fromBacillus stearothermophlus which affect cyclization characteristics and thermostability. J. Bacteriol., 174: 7478–7481.
Gawande B.N., Goel A., Patkar A.Y., Nene S.N. (1999). Purification and properties of a novel raw starch degrading cyclomaltodextrin glucanotransferase fromBacillus firmus. Appl. Microbiol. Biotechnol., 51: 504–509.
Harata K., Haga K., Nakamura A., Aoyagi M., Yamame K. (1996). X-ray structure of cyclodextrin glucanotransferase from alkalophilicBacillus sp. 1011. Comparison of two independent molecules at 1.8 Å resolution. Acta Crystallogr. D, 52: 1136–1145.
Hedges R.A. (1998). Industrial applications of cyclodextrins. Chem. Rev., 98: 2035–2044.
Higuti I.H., Grande S.W., Sacco R., Jose do Nascimento A. (2003). Isolation of alkalophilic CGTase-producing bacteria and characterization of cyclodextrin-glycosyltransferase. Brazilian Arch. Biol. Technol., 46: 1–7.
Horikoshi K. (1999). Alkaliphiles: Some applications of their products for biotechnology. Microbiol. Mol. Biol. Rev., 63 (4): 735–750.
Kaneko T., Kato T., Nakamura N., Horikoshi K. (1987). Spectrophotometric determination of cyclization activity of β-cyclodextrin-forming cyclomaltodextrin glucanotransferase. J. Japan. Soc. Starch Sci., 34: 45–48.
Li D.Q., Ma M. (2000). Nanosponges for water purification. Clean Products and Processes, 2: 112–116.
Marechal L.R., Rosso A.M., Marechal M.A., Krymkiewicz N., Ferrarotti S.A. (1996). Some properties of a cyclodextrin-glucanotransferase fromBacillus circulans DF 9 R type. Cell. Mol. Biol., 42: 659–664.
Martins R.F., Hatti-Kaul R. (2002). A new cyclodextrin glycosyltransferase from an alkalophilicBacillus agaradhaerens isolate: purification and characterization. Enzyme Microb. Technol., 30: 116–124.
Park H.T., Shin H.D., Lee Y.H. (1999). Characterization of the β-cyclodextrin glucanotransferase gene ofBacillus firmus var.alkalophilus and its expression inE. coli. J. Microbiol., 9: 811–819.
Sabioni J.G., Park Y.K. (1992). Cyclodextrin glycosyltransferase production by alkalophilicBacillus lentus. Rev. Microbiol., 23: 128–132.
Sian H.K., Said M., Hassan O., Kamaruddin K., Ismail A.F., Rahman R.A., Mahmood N.A.N., Illias R.M. (2005). Purification and characterization of cyclodextrin glucanotransferase from alkalophilicBacillus sp. G1. Proc. Biochem., 40: 1101–1111.
Sin K.A., Nakamura A., Kobayashi K., Masaki H., Uozumi T. (1991). Cloning and sequencing of a cyclodextrin glucanotransferase gene fromBacillus ohbensis and its expression inEscherichia coli. Appl. Microbiol. Biotechnol., 35: 600–605.
Singh M., Sharma R., Banerjee U.C. (2002). Biotechnological applications of cyclodextrins. Biotechnol. Adv., 20: 341–359.
Szejtli J. (1997). Utilization of cyclodextrins in industrial products and processes. J. Material Chem., 7: 575–587.
Terada Y., Yanase M., Takata H., Takaha T., Okada S. (1997). Cyclodextrins are not the major cyclic α-1,4-glucans produced by the initial action of cyclodextrin glucanotransferase on amylose. J. Biol. Chem., 272 (25): 15729–15733.
Tonkova A. (2006). Microbial starch converting enzymes of the α-amylase family. In: Ray R.C., Ward O.P., Eds, Microbial Biotechnology in Horticulture, Vol. 1, Science Publishers, Enfield, New Hampshire, USA, pp. 421–472.
Uitdehaag J.C.M., van Alebeek G., Van der Veen B.A., Dijkhuizen L., Dijkstra B.W. (2000). Structures of maltohexaose and maltoheptaose bound at the donor sites of cyclodextrin glycosyltransferase give insight into the mechanisms of transglycosylation activity and cyclodextrin size specificity. Biochemistry, 39: 7772–7780.
Uitdehaag J.C.M., van der Veen B.A., Dijkhuizen L., Dijkstra B.W. (2002). Catalytic mechanism and product specificity of cyclodextrin glycosyltransferase, a prototypical transglycosylase from the α-amylase family. Enzyme Microb. Technol., 30: 295–304.
Van der Veen B.A., Uitdehaag J.C.M., Dijkstra B.W., Dijkhuizen L. (2000). Engineering of cyclodextrin glycosyltransferase reaction and product specificity. Biochim. Biophys. Acta, 1543: 336–360.
Vassileva A., Burhan N., Beschkov V., Ivanova V., Tonkova A. (2003a). Immobilization ofBacillus circulans ATCC 21783 cells for cyclodextrin glucanotransferase production. In: Proceeding of the XIth International Workshop on Bioencapsulation, Strasbourg, France, 25–27 May 2003, pp. 201–204.
Vassileva A., Burhan N., Beschkov V., Spasova D., Radoevska S., Ivanova V., Tonkova A. (2003b). Cyclodextrin glucanotransferase production by free and agar gel immobilized cells ofBacillus circulans ATCC 21783. Proc. Biochem., 38: 1585–1591.
Vassileva A., Beschkov V., Ivanova V., Tonkova A. (2005). Continuous cyclodextrin glucanotransferase production by free and immobilized cells ofBacillus circulans ATCC 21783 in bioreactors. Proc. Biochem., 40: 3290–3295.
Yim D.E., Sato H.H., Park Y.H., Park Y.K. (1997). Production of cyclodextrin from starch by cyclodextrin glycosyltransferase fromBacillus firmus and characterization of purified enzyme. J. Ind. Microbiol. Biotechnol., 18: 402–405.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vassileva, A., Atanasova, N., Ivanova, V. et al. Characterisation of cyclodextrin glucanotransferase fromBacillus circulans ATCC 21783 in terms of cyclodextrin production. Ann. Microbiol. 57, 609–615 (2007). https://doi.org/10.1007/BF03175362
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF03175362