Abstract
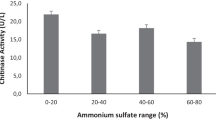
Metarhizium anisopliae, an entomopathogenic hyphomycete, is being used effectively in Integrated Pest Management (IPM) system. Foliar application of these fungi is quite satisfactory as it invades its host by adhering to insect cuticles and formation of penetration structures called appresoria, which produces various extracellular enzymes, including chitinase that causes the insect cuticle breaching. The induction and repression mechanism of chitinase activity is not entirely understood and activity of this enzyme is different in response to different carbon and nitrogen sources. This report illustrates the effect of two carbon sources viz. colloidal chitin and dextrose and a nitrogen source, yeast extract on the chitinase production of fourteenM. Anisopliae isolates. The chitinase activity varied among the isolates and the different media used. A high enzymatic activity was observed in the medium containing an extra nitrogen source (yeast extract) followed by the medium containing colloidal chitin as a sole source of carbon and nitrogen. The exochitinase activity and the chitinase activity gel were also studied for the isolates showing high chitinase enzyme production. An array of chitinase isozymes were observed on chitinase activity gel with a common 14.3 kDa enzyme for all the isolates.
Similar content being viewed by others
References
Anderson S.O. (1979). Biochemistry of insect cuticle. Ann. Rev. Entomol., 24: 29–61.
Bajan C., Kalalova S., Samsinakova A., Wojciechowska M. (1979). The relationship between infectious activities of entomopathogenic fungi and their production of enzymes. Bull. Acad. Pol. Sci. Cl. 2 Ser. Sci. Biol., 27: 963–968.
Barreto C.C., Charley C.S, Schrank A., Vainstein M.H. (2004). Distribution of chitinases in the entomopathogenMetarhizium anisopliae and effect ofN-acetyl glucosamine in protein secretion. Curr. Microbiol., 48 (2): 102–107.
Bradford M.M. (1976). A rapid and sensitive method for quantification of microgram quantities of protein utilizing the principle of protein dye binding. Anal. Biochem., 72 (1–2): 248–254.
Campos R.A, Arruda W., Boldo J.T., De silva M.V., De Barros N.M., De Azevado J.L., Schrank A, Vainstein M.H. (2005).Boophilus microplus infection byBeauveria amorpha andBeauveria bassiana: SEM analysis and regulation of subtilisin-like proteases and chitinases. Curr. Microbiol., 50 (5): 257–261.
Charnley A.K., St. Leger R.J. (1991). The role of cuticle degrading enzymes in fungal pathogenesis in insects. In: Cole G.T., Hoch M.C., Eds, The Fungal Spore and Disease Initiation in Plants and Animals. Plenum, New York, pp. 267–286.
Cooper R.M., Wood R.K.S (1975). Regulation of synthesis of cell wall degrading enzymes byVerticillium albo-atrum andFusarium oxysporum f. sp.lycopersice. Physiol. Plant Pathol., 5 (2): 135–156.
Coudron T.A., Kroha M.J., Ignoffo C.M. (1984). Levels of chitinolytic activity during development of three entomopathogenic fungi. Comp. Biochem. Physiol., 79 (3): 339–348.
Cruz De la J., Manuel R., Lora J.M., Gallego A.H., Dominguez F., Toro J.A.P., Antonio L., Benitez T. (1993). Carbon source control on glucanases, chitobiase and chitinase fromTrichoderma harzianum. Arch. Microbiol., 159 (4): 316–322.
De Moraes C.K., Schrank A., Vainstein M.H. (2003). Regulation of extracellular chitinases and proteases in the entomopathogen and acaricideMetarhizium anisopliae. Curr. Microbiol. 46 (3):205–221.
Deutscher J. (2008). The mechanisms of carbon catabolite repression in bacteria. Curr. Opin. Microbiol., 11 (2): 87–93.
El-Sayed G.N., Coudron T.A., Ignoffo C.M., Riba G. (1989). Chitinolytic activity and virulence associated with native and mutant isolates of an entomopathogenic fungus,Nomuraea rileyi. J. Invertebr. Pathol., 54 (3): 394–403.
Fang W., Leng B., Xiao Y., Jin K., Ma J., Fan Y., Feng J., Yang X., Zhang Y., Pei Y. (2005). Cloning ofBeauveria bassiana chitinase geneBbchit1 and its application to improve fungal strain virulence. Appl. Environ. Microbiol., 71 (1): 363–370.
Gabriel B.P. (1968). Enzymatic activities of some entomopathogenous fungi. J. Invertebr. Pathol., 11 (1): 70–81.
Inglis D.G., Goettel M.S., Butt T.M., Strasser H. (2001). Use of hyphomyecete fungi for managing insect pests. In: Butt T.M., Jackson C.W., Magan N., Eds; Fungi as Biocontrol Agents-Progress Problems and Potential. 1st edn., CAB International, Wallingford, UK, pp. 23–69.
Kang S.C., Park S., Lee D.G. (1999). Purification and characterization of a novel chitinase from the entomopathogenic fungus,Metarhizium anisopliae. J. Invertebr. Pathol., 73: 276–281.
Lopes M.A., Gomes D.S., Bello Koblitz M.G., Pirovani C.P., de Mattos Cascardo J.C., Goes-Neto A., Micheli F. (2008). Use of response surface methodology to examine chitinase regulation in the basidiomyceteMoniliophtora perniciosa. Mycol. Res., 112 (3): 399–406.
Muzzarelli M. (1999). Native, industrial and fossil chitins. In: Jolles P., Muzzarelli R., Eds, Chitin and Chitinases, Birkhauser, Basel.
Nawani N.N., Kapadnis B.P. (2005). Optimization of chitinase production using statistics based experimental designs. Process Biochem., 40 (2): 651–660.
Omero C., Benjamin A., Horwitz, Chet I. (2001). A convenient fluorometric method for the detection of extracellularN-acetylglucosaminidase production by filamentous fungi. J. Microbiol. Methods, 43 (3): 165–169.
Pegg G.F., Young D.H. (1982). Purification and characterization of chitinase enzymes from healthy andVerticillium alboatrum infected tomato plants and fromV. Alboatrum. Physiol. Plant Pathol., 21 (3): 389–409.
Reissig J.L., Strominger J.L, Leloir L.F. (1955). A modified colorimetric method for the estimation of n-acetyl amino sugars. J. Biol. Chem., 959–966.
Ronne H. (1995). Glucose repression in fungi. Trends Genet., 11 (1): 12–17.
Sandhya C., Krishna Adapa L., Madhavan Nampoothiri K., Binod P., Szakacs G., Pandey A. (2004). Extracellular chitinase production byTrichoderma harzianum in submerged fermentation. J. Basic Microbiol., 44 (1): 49–58.
Simahara K., Takiguchi Y. (1988). Preparation of crustacean chitin. Methods Enzymol., 161: 417–423.
St. Leger R.J., Cooper R.M., Charnley A.K. (1986). Cuticle-degrading enzymes of entomopathogenic fungi: Regulation of production of chitinolytic enzymes. J Gen Microbiol., 132: 1509–1517.
St. Leger R.J., Staples R.C., Roberts D.W. (1993). Entomopathogenic isolates ofMetarhizium anisopliae, Beauveria bassiana, Aspergillus flavus produce multiple extracellular chitinase isozymes. J. Invertebr. Pathol., 61 (1): 81–84.
St. Leger R.J., Joshi L., Bidochka M.J., Rizzo N.W., Roberts D.W. (1996). Biochemical characterization and ultra structural localization of two extracellular trypsins produced byMetarhizium anisopliae in infected insect cuticles. Appl. Environ. Microbiol., 62 (4): 1257–1264.
St. Leger R.J., Joshi L., Roberts D.W. (1998). Ambient pH is a major determinant in the expression of cuticle degrading enzymes and hydrophobin byMetarhizium anisopliae. Appl. Environ. Microbiol., 64 (2): 709–713.
Tamayo E.N., Villanueva A., Hasper A.A., De Graff L.H., Ramon D., Orejas M. (2008). CreA mediates repression of the regulatory gene xlnR which controls the production of xylanolytic enzymes inAspergillus nidulans. Fungal Genet. Biol., 45 (6): 984–993.
Trudel J., Asselin A. (1989). Detection of chitinase activity after polyacrylamide gel electrophoresis. Anal. Biochem., 178 (2): 362–366.
Yanagita T. (1980). The formaldehyde resistance ofAspergillus fungi attacking silkworm larvae: the relationship between pathogenicity to silkworm larvae and chitinase activity ofAspergillus flavus oryzae. J. Seric. Jpn., 49: 440–445.
Yanai K., Takaya N., Kojima N., Horiuchi H., Ohta A., Takagi M. (1992). Purification of two chitinases fromRhizopus oligosporus and isolation and sequencing of the encoding genes. J. Bacteriol., 174 (22): 7398–7406.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dhar, P., Kaur, G. Effects of carbon and nitrogen sources on the induction and repression of chitinase enzyme fromMetarhizium anisopliae isolates. Ann. Microbiol. 59, 545–551 (2009). https://doi.org/10.1007/BF03175144
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF03175144