Summary
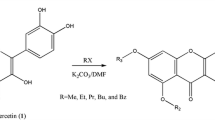
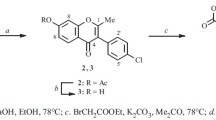
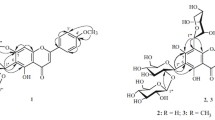
A method of complete synthesis of 5∶6∶7∶8-hydroxy flavonols is described. It starts from ω∶3∶6-trimethoxy-2∶4-dihydroxy-acetophenone which is subjected to partial methylation (of the 4-hydroxyl group) and subsequently to persulphate oxidation. The product, 2∶5-dihydroxy-ω∶3∶4∶6-tetramethoxy-acetophenone is condensed with the anhydride and sodium salt of anisic acid and also of benzoic acid. The resulting 6-hydroxy-flavones yield on further methylation the fully methylated ethers of calycopteretin and 6∶8-dihydroxy-galangin and on demethylation, the free hydroxy-flavonols.
Similar content being viewed by others
References
Venkateswarlu and SeshadriProc. Ind. Acad. Sci., A, 1946,23, 192.
Baker, Nodzu and RobinsonJ.C.S., 1929, 77.
Rao, Rao and SeshadriProc. Ind. Acad. Sci., A, 1944,19, 88.
PerkinJ.C.S., 1913,103, 650.
Rao and SeshadriProc. Ind. Acad. Sci., A, 1945,22, 162.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Murti, V.V.S., Row, L.R. & Seshadri, T.R. 5∶6∶7∶8-hydroxyflavonols. Proc. Indian Acad. Sci. (Math. Sci.) 24, 233 (1946). https://doi.org/10.1007/BF03174757
Received:
DOI: https://doi.org/10.1007/BF03174757