Abstract
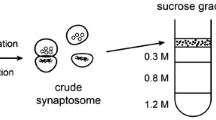
Studies on the molecular basis of neurological and psychiatric disorders often rely on the precise determination of specific proteins in brain tissues. In this study, we have developed a method for measuring the levels of the neural-specific growth-associated protein, GAP-43, in human postmortem brain specimens. This rapid and quantitative method is based on immunodetection procedures. Briefly, synaptosomal plasma membranes (SPMs) are deposited onto polyvinylidene difluoride (PVDF) membranes via a dot-blotting apparatus, followed by specific GAP-43 detection using a monospecific polyclonal antibody. Overall, the dot-blot procedure provided several advantages over Western blots and one-dimensional and two-dimensional polyacrylamide gels. The assays were more sensitive, reproducible, and allowed the rapid and simultaneous determination of multiple samples. Using this technique, we examined the levels of the GAP-43 protein in Brodmann’s areas 17, 20, and 10 of schizophrenic and age-, sex- and postmortem interval (PMI) matched controls. These studies revealed an increase in the levels of GAP-43 in visual association and frontal cortices (areas 20 and 10) of schizophrenic brains. Given the relationship of GAP-43 expression with the establishment and remodeling of neural connections, our results support the hypothesis that schizophrenia is associated with a perturbed organization of synaptic connections in associative areas of the human brain.
Similar content being viewed by others
Abbreviations
- GAP-43:
-
43-kDa growth-associated protein
- PMI:
-
postmortem interval
- SPM:
-
synaptosomal plasma membrane
- PVDF:
-
polyvinylidene difluoride
References
Benowitz L. I. and Routtenberg A. (1987) A membranc phosphoprotein associated with neural development, axonal regeneration, phospholipid metabolism, and synaptic plasticity.Trends Neurosci. 1, 527–532.
Benowitz L. I., Perrone-Bizzozero N. I., Finklestein S. P., and Bird E. D. (1989) Localization of the growth-associated phosphoprotein GAP-43 (B-50, F1) in the human cerebral cortex.J. Neurosci. 9, 990–995.
Benowitz L. I., Apostolides P. J., Perrone-Bizzozero N. I., Finklestein S. P., and Zwiers H. (1988) Anatomical distribution of the growth-associated protein GAP-43/B50 in the adult rat brain.J. Neurosci. 8, 339–352.
Blake M. S., Johnston K. H., Russell-Jones G. J., and Gotschlich E. C. (1984) A rapid, sensitive method for detection of alkaline phosphatase-cojjugate anti-antibody on Western blots.Anal. Biochem. 136, 175–179.
Bardford M. (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding.Anal. Biochem. 72, 248–254.
Laemmli U. K. (1970) Cleavage of structural proteins during assembly of the head of bacteriophage T4.Nature 227, 680–685.
Neve R. L., Perrone-Bizzozero N. I., Finklestein S. P., Zwiers H., Bird E., Kurnit D. M., and Benowitz L. I. (1987) The neuronal growth-associated protein GAP-43 (B-50, F1): neuronal specificity, developmental regulation and regional distribution of the human and rat mRNAs.Mol. Brain Res. 2, 177–183.
Neve R. L., Finch E. A., Bird E. D., and Benowitz L. I. (1988) The growth-associated protein GAP-43 (B-50, F1) is expressed selectively in associative regions of the adult human brain.Proc. Natl. Acad. Sci. USA 85, 3638–3642.
Nguyen T. V., Kosofsky B. E., Birnbaum R., Cohen B. M., and Hyman S. E. (1992) Differential expression of c-Fos and Zif268 in rat striatum after haloperidol, clozapine, and amphetamine.Proc. Natl. Acad. Sci. USA 89, 4270–4274.
Perrone-Bizzozero N. I., Weiner D., Hauser G., and Benowitz L. I. (1988) Extraction of major acidic Ca2+ dependent phosphoproteins from synaptic membranes.J. Neurosci. Res. 20, 346–350.
Perrone-Bizzozero N. I., Benowitz L. I., Bird E. D., Lewis S. E., Boling L., Holtzman P., and Matthysse S. (1991) Increased phosphorylation of GAP-43 and other synaptic proteins in schizophrenic brains.Soc. Neurosci. Abstr. 17, 1455.
Skene J. H. P. (1989) Axonal growth-associated proteins.Annu. Rev. Neurosci. 12, 127–156.
Towbin J., Staehelin T., and Gordon J. (1979) Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: Procedure and some applications.Proc. Natl. Acad. Sci. USA 76, 4350–4354.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Sower, A.C., Bird, E.D. & Perrone-Bizzozero, N.I. Increased levels of GAP-43 protein in schizophrenic brain tissues demonstrated by a novel immunodetection method. Molecular and Chemical Neuropathology 24, 1–11 (1995). https://doi.org/10.1007/BF03160108
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF03160108