Abstract
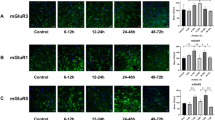
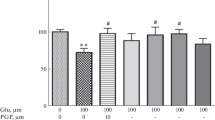
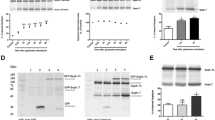
As an in vitro model of CNS excitatory amino acid (EAA) injury, rat cortical neuronal cultures were challenged with glutamate (0.5 or 10 mM) and the levels of released lactate dehydrogenase (LDH) were monitored at 1 h, 1, 2, and 7 d. LDH release is correlated with levels of plasma membrane damage. GM1 has been shown to be continuously distributed on the outer surface of CNS cellular membranes. By staining for the distribution of endogenous GM1 ganglioside using cholera toxin/antitoxin immunohistochemistry, we were able to assess morphologically cellular plasma membrane integrity after damage. We used these two measures (LDH and GM1 localization) to study the neuroprotective effects of exogenous GM1 ganglioside to further elucidate its mechanism. Cortical cultures derived from 15-d rat fetuses were subjected to the glutamate challenge for 30 min. Parallel cultures were either pre- or posttreated with 80 μM of GM1. Exposure to 10 mM glutamate caused a highly significant increase in LDH release at 1–48 h. Pretreatment with GM1 reduced the release, whereas post-treatment reduced the LDH release even more. Plasma membrane changes observed by the GM1 immunohistochemistry reflected the LDH release data. All cultures treated with GM1 evidenced substantial structural integrity (continuous staining of GM1 along perykarya and processes) as compared to untreated cultures. These data support our hypothesis that GM1 treatment (pre- and post-) reduces plasma membrane damage.
Similar content being viewed by others
References
Asou H., Mutou N., Hirano S., Takagi T., Kajiwara K., Komatsubara J., and Sugaya E. (1986) Ganglioside GM1 localization in cholinergic neurons visualized by use of a double avidin-biotin complex system.Cell Struct. and Function 11, 175–180.
Bonheur J., Laev H., Vorwerk C., and Karpiak S. E. (1993) Traumatic injury of spinal cellsin vitro reduced by GM1 ganglioside.Restorative Neurology and Neuroscience. in press.
Choi D. W. (1985) Glutamate toxicity in cortical cell culture is calcium dependent.Neurosci. Lett. 58, 293–297.
Choi D. W. (1987) Ionic dependence of glutamate toxicity.J. Neurosci. 7, 369–379.
Choi D. W., Maulucci-Gedde M. A., and Kriegstein A. R. (1987) Glutamate neurotoxicity in cortical cell culture.J. Neurosci. 7, 357–368.
Choi D. W. (1988) Glutamate toxicity and diseases of the nervous system (A Review).Neuron 1, 623–634.
Choi D. W., Koh J., and Peters S. (1988) Pharmacology of glutamate neurotoxicity in cortical cell-culture: Attenuation by NMDA antagonists.J. Neurosci. 8, 185–196.
Choi D. W. and Rothman S. M. (1990) The role of glutamate neurotoxicity in hypoxic-ischemic neuronal death.Ann. Rev. Neurosci. 13, 171–182.
Cuatrecasas P. (1973) Gangliosides and membrane receptors for cholera toxin.Biochemistry 12, 3558–3566.
Dichter M. A. (1978) Rat cortical neuron cell culture: Culture methods, cell morphology, electrophysiology, and synapse formation.Brain Res. 149, 279–293.
Facci L., Leon A., and Skaper S. D. (1990a) Hypoglycemic neurotoxicity in vitro: Involvement of excitatory amino acid receptors and attenuation by monosialoganglioside GM1.Neuroscience 37, 709–716.
Facci L., Leon A., and Skaper S. D. (1990b) Excitatory amino acid neurotoxicity in cultured retinal neurons. Involvement of N-methyl-D-aspartate (NMDA) and non-NMDA receptors and effect of ganglioside GM1.J. Neurosci. Res. 27, 202–210.
Favaron M., Manev H., Alho H., Bertolino M., Ferret B., Guidotti A., and Costa E. (1988) Gangliosides prevent glutamate and kainate neurotoxicity in primary neuronal cultures of neonatal rat cerebellum and cortex.Proc. Nat. Acad. Sci. USA 85, 7351–7355.
Favaron M., Manev H., Vicini S., Guidotti A., and Costa E. (1990) Prevention of excitatory amino acid-induced neurotoxicity by natural and semi-synthetic sphingolipids, inNeurotoxicity of Excitatory Amino Acids (Guidotti A., ed.), pp. 243–258, Raven, New York.
Goldberg M. P., Moyner H., and Choi D. W. (1988) Hypoxic neuronal injuryin vitro depends on extracellular glutamine.Neurosci. Lett. 94, 52–57.
Hakomori S. I. (1981) Glycosphingolipids in cellular interactions, differentiation and oncogenesis.Annu. Rev. Biochem. 50, 733–764.
Hansson H. A., Holmgren J., and Svennerholm L. (1977) Ultrastructural localization of cell membrane ganglioside by cholera toxin.Proc. Natl. Acad. Sci. USA 74, 3782–3786.
Hertz E., Yu A. C. H., Hertz L., Juurlink B. H. J., and Schousboe A. (1989) Preparation of primary cultures of mouse cortical neurons, inA Dissection and Tissue Culture Manual of the Nervous System (Shakar A., et al., eds.), pp. 183–186, Liss, New York.
Hungund B. L. and Mahadik S. P. (1981) Topographic studies of gangliosides of intact synaptosomes from brain cortex.Neurochem. Res. 6, 183–191.
Kanda S., Inoue K., Nojima S., Utaumi H., and Wiegandt H. (1982) Incorporation of spin-labelled ganglioside analogues into cell and liposomal membranes.J. Biochem. 91, 1707–1717.
Karpiak S. E., Li Y. S., and Mahadik S. P. (1986) Gangliosides (GM1 and AGF2) reduce mortality due to ischemia: Protection of membrane function.Stroke 18, 184–187.
Karpiak S. E., Mahadik S. P., and Wakade C. G. (1990) Ganglioside reduction of ischemic injury.Crit. Rev. Neurobiol. 5, 221–237.
Karpiak S. E., Wakade C. G., Tagliavia A., and Mahadik S. P. (1991a) Temporal changes in edema, Na+, K+ and Ca++ in focal cortical stroke: GM1 ganglioside reduces ischemic injury.J. Neurosci Res. 30, 512–520.
Karpiak S. E., Ortiz A., Wakade C. G., Hernandez N., Durkin M., Barkai A. I., and Mahadik S. P. (1991b) Primary and secondary injury processes in stroke: High45Ca++ levels and Ca++-ATPase losses reduced by GM1 ganglioside.Abstr. Soc. Neurosci. 16, 278.
Kennedy P. G. E. (1982) Neural cell markers and their applications to neurology.J. Immunol. 2, 35–53.
Kim S. H., Moretto G., Lee V., and Yu R. K. (1986) Neuroimmunology of gangliosides in human neurons and glial cells in culture.J. Neurosci. Res. 15, 303–321.
Koh J. and Choi D. W. (1987) Quantitative determination of glutamate mediated cortical neuronal injury in cell culture by lactate dehydrogenase efflux assay.J. Neurosci. Methods 20, 83–90.
Koh J., Goldberg M. P., Hartley D. M., and Choi D. W. (1990) Non-NMDA receptor-mediated neurotoxicity in culture.J. Neurosci. 10, 693–705.
Laev H. and Mahadik S. P. (1989) Topography of monosialoganglioside (GM1) in rat brain using monoclonal antibodies.Neurosci. Lett. 102, 7–14.
Laev H., Williams B., Bonheur J., Karpiak S. E., and Mahadik S. P. (1991) Topographical exposure of endogenous GM1 after glutamate toxicity: Exogenous GM1 protective effects.Abstr. Soc. Neurosci. 17, 787.
Ledeen R. W. (1978) Gangliosides structure and distribution: are they located at the nerve ending?J. Supramol. Struct. 8, 1–17.
Ledeen R. W. (1985) Biology of gangliosides: Neurogenetic and neuronotrophic properties.J. Neurosci. Res. 12, 147–159.
Leon A., Bevegnu D., Dal Toso R., Giorgio O., Presti D., Tettamanti G., and Toffano G. (1986) Neuronal cell cultures and monosialoganglioside: a model for comprehension of mechanisms underlying central nervous system repair.Exp. Brain. Res. 513, 215–224.
Leon A., Dal Toso R., Presti D., Bevegnu D., Facci L., Kirschner G., Tettamanti G., and Toffano G. (1988) Development and survival of neurons in dissociated fetal mesencephalic serum-free cultures: II Modulatory effects of gangliosides.J. Neurosci. 8, 746–753.
Leskawa K. C., Erwin E., Leon A., Toffano G., and Hogan E. L. (1989) Incorporation of exogenous ganglioside GM1 into neuroblastoma membranes: Inhibition by calcium ion and dependence upon membrane protein.Neurochem. Res. 14, 547–554.
Mahadik S. P., Hungund B. L., and Rapport M. M. (1978) Topographic studies of glycoproteins of intact synaptosomes from rat brain cortex.Biochim. Biophys. Acta 511, 240–250.
Mahadik S. P. and Karpiak S. E. (1988) Gangliosides in treatment of neuronal injury and disease.Drug Dev. Res. 15, 337–360.
Mahadik S. P., Hawyer D. B., Hungund, B. L., Li Y. S., and Karpiak S. E. (1989) GM1 ganglioside treatment after global ischemia protects changes in membrane fatty acids and properties of (Na++K+)-ATPase and Mg++-ATPase.J. Neurosci. Res. 24, 402–412.
Mahadik S. P. (1992) Gangliosides, new generation of neuroprotective agents, inEmerging Strategies in Neuroprotection (Marangos P. and Lal H., eds.), pp. 187–223, Burkhauser, Boston, MA.
Mahadik S. P., Bharucha V. A., Stadlin A., Ortiz A., and Karpiak S. E. (1992) Loss and recovery of alpha+ and alpha isozymes of (Na++K+)-ATPase in cortical focal ischemia: GM1 ganglioside protects plasma membrane structure and function.J. Neurosci. Res. 32, 209–220.
Mahadik S. P., Makar T. K., Murthy J. N., Ortiz A., Wakade C. G., and Karpiak S. E. (1993a) Temporal changes in superoxide dismutase, glutathione peroxidase and catalase in primary and peri-ischemic tissue: Monosialoganglioside (GM1) treatment effects.Molec. Chem. Neuropath. 18, 1–14.
Mahadik S. P., Hungund B. L., Gokhale V. S., Ortiz A., Makar T. K., and Karpiak S. E. (1993b) Monosialoganglioside (GM1) restores membrane fatty acid levels in ischemic tissue after focal ischemia rat.Neurochem. Intl. 23, 162–172.
Manev H., Favaron M., Guidotti A., and Costa E. (1989) Delayed increase of Ca++ influx elicited by glutamate: Role in neuronal death:Mol. Pharmacol. 36, 106–112.
Manev H., Favaron M., Vicini S., and Guidotti A. (1990a) Ganglioside-mediated protection from glutamate-induced neuronal death.Acta Neurobiol. Exp. 50, 475–488.
Manev H., Favaron M., Vicini S., Guidotti A., and Costa E. (1990b) Glutamate-induced neuronal death in primary cultures of cerebellar granule cells: Protection by synthetic derivatives of endogenous sphingolipids.J. Pharmacol. Exp. Ther. 252, 419–427.
Masco D., Flott B., and Seifert W. (1989) Astrocytes in cell culture incorporate GM1 ganglioside.Glia 2, 231–240.
Mazzari S., Karpiak S. E., Lipartiti M., Seren S., Lazzaro A., Rubin A., Kogan T., Fadda E., Toffano G., and Leon A. (1990) Monosialoganglioside effects on excitatory amino acid-related neurotoxicity and cerebral ischemia, inNeurotoxicity of Excitatory Amino Acids (Guidotti A., ed.), pp. 281–291, Raven, New York.
Murphy T. H., Schnaar R. L., and Coyle J. T. (1990) Immature cortical neurons are sensitive to glutamate toxicity in inhibition of cysteine uptake.FASEB J. 4, 1624–1632.
Orlando P., Cocciante G., Ippolito G., Massari P., Roberti S., and Tettamanti G. (1979) The fate of tritium labelled GM1 ganglioside injected in mice.Pharmacol. Res. Commun. 11, 759–773.
Raff M. C., Fields K. L., Hakomori S. I., Mirski R., Pruss R. M., and Winter J. (1979) Cell-type specific markers for distinguishing and studying neurons and the major class of glial cells in culture.Brain Res. 174, 283–308.
Rahman H. (1983) Functional implications of gangliosides in synaptic transmissions.Neurochem. Intl. 5, 539–547.
Robertson B. and Grant G. (1989) Immunocytochemical evidence for the localization of the GM1 ganglioside in carbonic-anhydrase containing RT97 immunoreactive rat primary sensory neurons.J. Neurocytol. 18, 77–86.
Roisen F. J., Bartfeld H., Nagele R., and Yorke F. (1981) Ganglioside stimulation of axonal sproutingin vitro.Science 214, 577–578.
Rothman S. M. and Olney J. W. (1986) Glutamate and the pathophysiology of hypoxic-ischemic brain damage.Ann. Neurol. 19, 105–111.
Schachner M. (1982) Cell surface antigens in the mammalian nervous system.J. Neurochem. 39, 1–8.
Schengrund C. L. (1990) The role(s) of gangliosides in neuronal differentiation and repair: a perspective.Brain Res. Bull 24, 131–141.
Skaper S. D., Leon A., and Toffano G. (1989a) Ganglioside function in the development and repair of the nervous system: From basic research to clinical application.Mol. Neurobiol. 3, 173–199.
Skaper S. D., Facci L., Milani D., and Leon A. (1989b) Monosialoganglioside GM1 protects against anoxia induced neuronal deathin vitro.Exp. Neurol. 106, 297–305.
Svennerholm L. (1964) The gangliosides.J. Lipid Res. 5, 145–155.
Willinger M. and Schachner M. (1980) GM1 ganglioside as a “marker” for neuronal differentiation in mouse cerebellum.Dev. Biol. 74, 101–117.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Laev, H., Mahadik, S.P., Bonheur, J.L. et al. GM1 ganglioside reduces glutamate toxicity to cortical cells. Molecular and Chemical Neuropathology 20, 229–243 (1993). https://doi.org/10.1007/BF03160076
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF03160076