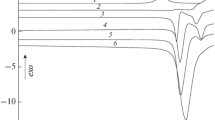
Abstract
The temperature-dependent specific electrical conductivity of polycrystalline, polymorphic NH4I samples was measured in the temperature range between −170°C and +25°C. The conductivity in the ranges of the tetragonal →CsCl-type and CsCl-type→NaCl type transformations can be explained by the formal kinetics of a diffusion-controlled nucleation and growth process. An estimation of the activation energy of the formation of the second-and first-phase nuclei in the respective parent phases by the evaluation of TTT curves yielded energy values of 0.004–0.05 and 0.0001–0.024 eV.
Резюме
Злектрическая проводимость, специфически зависяшая от температуры поликристалического и полиморфного образца NH4I была измерена в температурном интервале от −170°C до +25°C. Проводимость при переходах типа тетрагонального рCsCl и CsCl→NaCl можно обьяснить формальной кинетикой процессов возрастания и застроения под диффузионным контролом. Знергии активации образования ядер вторичной и первчной фаз в соотвестствушей исходной фазе определяется приближенно. Оцениваются TTT кривые, из которых значения знергии 0,004–0,05 и 0,001–0,024 зв соответственно.
Similar content being viewed by others
References
A. Smits andD. Tollenar, Z. phys. Chem.,B 52, 222, 1942.
V. Hovi andM. Varteva, Phys. kondens. Materie,3, 305, 1965.
H. P. Klug andW. W. Johnson, J. Amer. Chem. Soc.,59, 2061, 1937.
F. Simon, C. V. Simson andM. Ruhemann, Z. Phys. Chem.,129, 321, 1927.
R. G. W. Wickoff, Crystal Structures, Vol. I. Interscience Publishers, Inc., New York, 1948.
C. C. Stephenson, L. A. Landers andA. G. Cole, J. Chem. Phys.,20, 1044, 1952.
Z. Morlin, Acta Phys. Hung.,21, 137, 1966.
Z. Morlin, Acta Phys. Hung.,24, 277, 1968.
I. Gaál, Z. Morlin andI. Tarján, Acta Phys. Hung.,27, 25, 1969.
H. Weijma, Some Aspects of the Phase Transformation in Solid CsCl, V.R.B. Offsetdrukkerij, Groningen, 1971. (pp. VI, 85).
T. Morlin, Phys. stat. sol., (a)8, 565, 1971.
M. Avrami, J. chem. Phys.,7, 1103, 1939.
W. A. Johnson andR. F. Mehl, Trans. AIME135, 416, 1939.
J. W. Christian, The Theory of Transformation in Metals and Alloys, Pergamon Press, Oxford, 1965 (pp. XVII, 973).
J. Burke, The Kinetics of Phase Transformation in Metals, Pergamon Press, Oxford, 1965 (pp. VIII, 226).
P. Gordon andR. A. Vandermeer in Recrystallization, Grain Growth and Textures. Ed. by H. Margolin, Amer. Soc. Metals, Metals Park (Ohio), 1966, (pp. 205 to 266).
Author information
Authors and Affiliations
Additional information
Dedicated to Prof.I. Tarján on his 60th birthday.
Rights and permissions
About this article
Cite this article
Morlin, Z. Kinetics of the low-temperature phase transformation of ammonium iodide. Acta Physica 33, 377–385 (1973). https://doi.org/10.1007/BF03158073
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF03158073