Summary
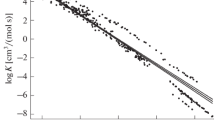
The alkaline hydrolysis of ethyl acetate in water containing small additions of acetone have shown that the earlier expectations of abnormal trend in the rate of reaction were correct. The absence of mechanistic change is indicated by the continuity in the curve for correlation of the two Arrhenius parameters. Any explanation has to be in relation to the structure of the environment of the reacting species and the requirement of the properly hydrated reacting ion.
Similar content being viewed by others
References
Th. AckermannDiscussions Farad. Soc., 1957,24, 180.
Dawson, H. M.et al. J.C.S., 1927, 2444.
Fairclough and Hinshelwood.Ibid. J.C.S., 1938, 236.
Tommila and HinshelwoodIbid. J.C.S., 1938, 1801.
—Acad. Sci. Fennicœ, 1952,2A (47).
Nair, P. M. and Anantakrishnan, S. V.Proc. Ind. Acad. Sci., 1950,32, 187.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Anantakrishnan, S.V., Anantaraman, A.V. Kinetic studies in ester hydrolysis. Proc. Indian Acad. Sci. 49, 86–95 (1959). https://doi.org/10.1007/BF03052828
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF03052828