Summary
The coupling of 5-hydroxyflavone with benzenediazonium chloride led to an azo dye which was different from the compound obtained by the Robinson flavone condensation on 2-acetyl-4-benzeneazoresorcinol and benzoic anhydride. The two compounds are shown to be the 8- and 6-benzeneazo derivatives of 5-hydroxyflavone respectively. The conversion of both the azo dyes into 5∶6-dihydroxyflavone is described.
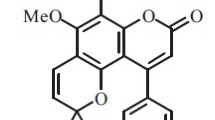
Tectochrysin was converted to baicalein 7-methyl ether by coupling with benzenediazonium chloride, followed by reduction of the azo dye to the amine and treatment of the latter with hydrochloric acid. Baicalein was obtained by demethylation of the 7-methyl ether. The same series of reactions carried out on 5-hydroxy-6-methoxyflavone25 led to 5∶6∶8-trihydroxyflavone.
Similar content being viewed by others
References
Iyer and VenkataramanProc. Ind. Acad. Sci., 1946,23A, 278.
SugasawaChemical Abstracts, 1938,32, 5833.
Wessely and co-workersMonatsh, 1930,56, 97; 1932,60, 26.
Baker, Brown and ScottJ. Chem. Soc., 1939, 1922.
Sastri and SeshadriProc. Ind. Acad. Sci., 1946,24A, 247.
BakerJ. Chem. Soc., 1939, 956.
Simonis and DanishevskiBer., 1926,59B, 2914.
SugasawaJ. Chem. Soc., 1934, 1483.
See also BakerIbid.,J. Chem. Soc., 1954.
Rajagopalan, Rao and SeshadriProc. Ind. Acad. Sci., 1947,25A, 432.
Ullal, Shah and WheelerJ. Chem. Soc., 1940, 1499.
Robinson and VenkataramanIbid.,J. Chem. Soc., 1926, 2344.
Späth and DobrovolnyBer., 1938,71B, 1831.
Sastri and SeshadriProc. Ind. Acad. Sci., 1946,23A, 273.
Shibata, Nakamura and IwataActa Phytochim., 1923,1, 105.
BargelliniGazzetta, 1919,49, II, 47.
Sastri and SeshadriProc. Ind. Acad. Sci., 1946,23A, 262.
Rajagopalan, Seshadri and VaradarajanIbid.,, 1950,31A, 31.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Iyer, R.N., Venkataraman, K. Synthetical experiments in the chromone group. Proc. Indian Acad. Sci. 37, 629–642 (1953). https://doi.org/10.1007/BF03052691
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF03052691