Abstract
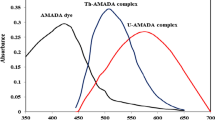
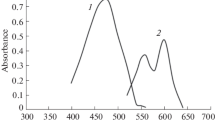
The spectrophotometric studies of uranium (VI)-pongamol complex have been carried out in 50% aqueous ethanolic solutions. Pongamol produces a yellow complex with an aqueous uranyl salt solution, the complex is soluble in 50% aqueous ethanol. The complex is quite stable for 24 hours, the optical density remaining constant at pH 5·6–7·1. The complex obeys Beer-Lambert’s law at 390 mµ in the concentration range of 1 to 5 p.p.m. of uranium in solution. The molar composition of the pongamol uranium (VI) complex has been found to be 2:1, and its tentative structure has been suggested. The cations and anions which interfere in the estimation of uranium using pongamol have been indicated.
Similar content being viewed by others
References
Rangaswami, S. and Seshadri, T. R.Ind. J. Pharm., 1941,3, 3–7.
Jatkar, S. K. K. and Mattoo, B. N.J. Ind. Chem. Soc., 1953,30, 779.
Vosburgh, W. C. and Cooper, G. R.J. Amer. Chem. Soc., 1941,63, 437.
Rangaswami, S. and Seshadri, T. R.Proc. Ind. Acad. Sci., 1949,15 A, 417.
Author information
Authors and Affiliations
Additional information
Communicated by Prof. T. R. Seshadri,f.r.s., f.a.sc.
Rights and permissions
About this article
Cite this article
Jain, B.D., Purushottam, K.V. Spectrophotometric studies of uranium (VI)-pongamol complex. Proc. Indian Acad. Sci. 64, 245–248 (1966). https://doi.org/10.1007/BF03049395
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF03049395