Abstract
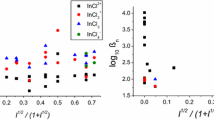
The standard rate constants for the reduction of europium in perchlorate and chloride media and the formal rate constants in sulphate, acetate and lactate solutions are reported. The rate increases in the order of increasing polarisability of the anion or the increasing stability of the complex,viz., perchlorate < sulphate > lactate ≈ acetate.
The effect of methanol on the polarography of europium has been studied. In perchlorate and chloride media, the half-wave potential shifts to negative values and then to positive values as the methanol content is increased. In acetate solutions, the shift is continuously to negative potentials. This is attributed to the effect of complex formation in acetate media and ion-pairing in perchlorate and chloride media. The effect of viscosity on the diffusion current was found to obey the Stokes-Einstein equation.
Similar content being viewed by others
References
Vlcek, A. A... Collection Czechoslov,Chem. Communs., 1955,20, 1507; 1959,24, 181;Chem. Listy, 1955,49, 565.
Kolthoff, I. M. and Coetzee, J. F.J. Amer. Chem. Soc., 1957,79, 1852.
Almagro, V. Almagro, J. and Sancho, J.Proc. Third Intern. Congr. Polarography, Southampton, Ed., G. J. Hills, Vol. 1, p. 667.
Coetzee, J. F. and Wei-San-Siao.Inorg. Chem., 1963,2, 14.
Greenough, M. L., Williams, Jr., W. E. and Taylor, J. K.Rev. Sci. Instr., 1951,22, 484.
Gierst, L. and Cornelissen, P. Collection Czechoslov,Chem. Communs., 1960,25, 3004.
Anderson, L. and Macero, D. J.J. Phys. Chem., 1963,67, 1942.
McCoy, H. N...J. Amer. Chem. Soc., 1936,58, 1579.
Koutecky, J... Collection Czechoslov,Chem. Communs., 1953,18, 597.
Randles, J. E. B...Canadian J. Chem., 1959,37, 238.
Sundaresan, R., Saraiya, S. C. and Sundaram, A. K.Proc. Ind. Acad. Sci., 1967,66, 120.
Randles, J. E. B. and Somerton, K. W.Trans. Faraday Soc., 1952,48, 937.
—..Progress in Polarography., Ed. P. Zuman and I. M. Kolthoff, Interscience Publishers, New York, 1962, p. 131.
Bjerrum, J., Schwarzenbach, G. and Sillen, L. G.Stability Constants, Part 1. ‘OrganicLigands,’ The Chemical Society Special Publication No. 6, 1957, p. 3.
-..Ibid., 1957, p. 12
Macero, D. J., Herman, H. B. and Dukat, A. J.Anal. Chem., 1965,37, 675.
Galaktinov, Yu. P. and Astakhov, K. V.Zh. Neorg. Khim., 1966,11, 1813.
Feakins, D...Physicochemical Processes in Mixed Aqueons Solvents, Heinemann Educational Books, London, 1966, p. 71.
Timmermans, J...Physicochemical Constants of Binary Systems in Concentrated Solutions, Interscience Publishers, New York, 1960,4, 168.
Shedlovsky, T. and Kay, R. L.J.Phys. Chem., 1956,60, 151.
Kolthoff, I. M. and Lingane, J. J.Polarography, Interscience Publishers, New York, 1946,1, 49.
—..ref. 19, p. 163.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Chandrasekaran, V.R., Sundaram, A.K. Polarography of europium in aqueous and aqueous methanolic solutions. Proc. Indian Acad. Sci. 74, 133–141 (1971). https://doi.org/10.1007/BF03047148
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF03047148