Summary
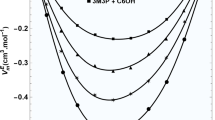
The kinetics of acid hydrolysis of ethyl acetate, ethyl propionate and ethyl chloracetate in glycerol-water system has been studied. Both viscosity and dielectric constant influence the reaction and a close correlation of the parameters of the reaction velocity equation is noticed.
Similar content being viewed by others
References
Evans and JenkinsTrans. Farad. Soc., 1940,36, 818.
Hinshelwoodet al. J. C. S., 1938,848, 862.
HinshelwoodKinetics of Chemical Change, (O.U.P., 1940), 251.
Hinshelwood and FaircloughJ. C. S., 1937, 538.
JowettPhil. Mag., 1929,8, 1059.
MenschutkinZ. Physik. Chem., 1890,6, 41.
Moelwyn-HughesKinetics of Reactions in Solution, (O.U.P, 1933),52, 107, 117, 158.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Anantakrishnan, S.V., Krishnamurti, S. Kinetic studies in ester hydrolysis. Proc. Indian Acad. Sci. (Math. Sci.) 14, 279–288 (1941). https://doi.org/10.1007/BF03046069
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF03046069