Summary
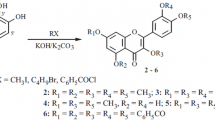
Partial methyl ethers of chrysin, apigenin, galangin and quercetin have been subjected to nuclear reduction in the 5-position through their tosyloxy compounds. The products are 7-methoxy flavone, pratol methyl ether, 3:7-dimethoxy flavone and fisetin tetramethyl ether respectively.
Similar content being viewed by others
References
Jain and Seshadri..J. Sci. Ind. Research (India), 1953,12B, 503.
Rao and Seshadri..Proc. Ind. Acad. Sci., 1943,18A, 232.
Murty and Seshadri.. Ibid., 1948,28A, 21.
Rao, Rao and Seshadri.. Ibid., 1947,25A, 430.
Mozingo..Org. Synthesis, 1941,21, 15.
——, Wolf, Harris and Folkers..J.A.C.S., 1943,65, 1015.
Robinson and Turner ..J.C.S., 1918, 876.
Tambor..Ber., 1916,49, 1710.
Allan and Robinson ..J.C.S., 1926, 2335.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Jain, A.C., Seshadri, T.R. Nuclear reduction in the 5-position of anthoxanthins. Proc. Indian Acad. Sci. (Math. Sci.) 38, 294–296 (1953). https://doi.org/10.1007/BF03045257
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF03045257