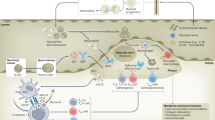
Abstract
Atherosclerosis is characterized as a chronic inflammatory-fibroproliferative disease of the vessel wall. The attachment of monocytes and T-lymphocytes to the injured endothelium followed by their migration into the intima is one of the first and most crucial steps in lesion development. The co-localization of CD4+T-cells and macrophages in the lesion, the abundant expression of HLA Class II molecules and the co-stimulatory molecule CD40 and its ligand (CD40L) indicate a contribution of cell-mediated immunity to atherogenesis. Transgenic mouse models revealed that dependent on the model T- and B-cells may promote lesion progression, monocytes and macrophages are in contrast essential for the development of atherosclerotic lesions. Apart from the local process in the vessel wall, systemic signs of an inflammatory reaction are also associated with lesion development. Thus plasma levels of C-reactive protein and fibrinogen and the white blood cell count are positively correlated to the risk of cardiovascular disease. Recently, an inflammatory phenotype of circulating peripheral blood monocytes could be demonstrated as a specific cellular correlate to lipid and lipoprotein risk factors. Thus the pool size of LPS receptor (CD14)dim and FcγIIIa receptor (CD16a)+ monocytes positively correlates to plasma cholesterol levels, to triglycerides levels and to the apolipoprotein E4 (apo E4) phenotype in contrast to a negative correlation to the high density lipoprotein (HDL) cholesterol concentration. This CD14dim CD16a+ monocytes are further characterized by a high expression of β1- and β2-integrins, suggesting a higher capacity for attachment at sites of inflammation. A proinflammatory cytokine pattern and an expansion of these cells in other inflammatory diseases are indicating that these cells promore the inflammatory process during atherogenesis. Surface expression of the activation antigen CD45RA on monocytes in correlation to plasma LDL cholesterol and Lp(a) levels further indicates an inflammatory reaction. Regarding the potential mechanisms of the phenotypic changes of peripheral blood monocytes, in a serum free in vitro differentiation model supplemented with M-CSF monocytes from probands which are homozygous for apo E4 showed a significantly higher increase of CD16a expression compared to apo E3/E3 cells indicating that a genetic polymorphism of a single apolipoprotiin gene locus may affect monocyte differentiation. The further characterization of the cellular immunology of monocytes and T-lymphocytes in lesion development will provide new specific diagnostic and therapeutic targets in atherogenesis.
Zusammenfassung
Die Arteriosklerose läßt sich als chronische, inflammatorisch-fibroproliferative Erkrankung der Gefäßwand charakterisieren. Als Antwort auf unterschiedliche Ursachen einer Endothelschädigung adhärieren zirkulierende Monozyten und T-Lymphozyten an das Endothel und wandern in die Gefäßintima. Die Kolokalisation von CD4+-T-Zellen und Makrophagen in der Läsion, die zahlreiche Expression von HLA-Klasse-II-Molekülen und des kostimulatorischen Moleküls CD40 und seines Liganden weisen darauf him, daß neben einer unspezifischen Inflammation auch eine spezifische zellvermittelte Immunantwort einen Beitrag zur Atherogenese leistet. Transgene Mausmodelle zeigen, daß in Abhängikeit des Modells T- und B-Lymphozyten die Entwicklung der arteriosklerotischen Läsionen verstärken können, im Unterschied zu Monozyten/Makrophagen aber nicht unbedingt notwending für deren Entwicklung sind. Neben dem lokal inflammatorischen Prozeß in der Gefäßwand sind auch systemische Zeichen einer entzündlichen Reaktion mit der Entwicklung der Arteriosklerose verbunden. So korrelieren Plasmaspiegel des C-reaktiven Proteins, des Fibrinogens und die Höhe der Leukozytenkonzentration positiv mit dem Risiko einer kardiovaskulären Erkrankung. Kärzlich konnte gezeigt werden, daß mit Lipid- und Lipoproteinrisikofaktoren der Atherogenese ein inflammatorischer Phänotyp der zirkulierenden Monozyten als spezifisches zelluläres Korrelat assoziiert ist. So zeigt die Populationsgröße der Monozyten, die den LPS-Rezeptor CD14 schwach und den FcγIIIa-Rezeptor CD16a stärker exprimieren, eine positive Korrelation mit der Plasmacholesterinkonzentration sowie mit dem Apolipoprotein-E4-Phänotyp und eine negative Korrelation zu HDL-Cholesterinwerten. Diese CD14schwach-CD16a+-Monozyten sind ferner durch eine hohe Expression von β1-and β2-Integrinen charakterisiert, was ein Hinweis auf eine stärkere Fähigkeit dieser Monozyten zur Adhärenz an entzündetes Gewebe sein könnte. Ein proinflammatorisches Zytokinmuster und eine Expansion dieser Zellen auch bei anderen entzündlichen Erkrankungen lassen vermuten, daß diese Zellen den entzündlichen Prozeß der Atherogenese weiter vorantreiben könnten. Ferner zeigt sich sowohl zum LDL-Cholesterin-als auch zum Lp(a)-Spiegel des Plasmas eine positive Korrelation der Expression des Aktivierungsantigens CD45RA auf allen Monozyten als Ausdruck einer entzündlichen Reaktion. Um potentielle Mechanismen dieser phänotypischen Veränderungen der Monozyten des peripheren Blutes weiter aufzudecken, wurde die Monozytendifferenzierung in einem serumfreien In-vitro-Differenzierungsmodell unter Zugabe von M-CSF analysiert. Hierbei war bei Monozyten von für das Apolipoprotein E4 homozygoten Spendern ein signifikant höherer Anstieg der CD16a-Expression im Vergleich zu Apo-E3/E3-Zellen zu beobachten, ein Zeichen, daß ein genetischer Polymorphismus eines einzelnen Apolipoprotein-Genlokus die Monozytendifferenzierung beiinflussen kann. Die weitere Charakterisierung der zellulären Immunologie von Monozyten und T-Lymphozyten in der Entwicklung von arteriosklerotischen Läsionen sollte neue spezifische Ansatzpunkte für die Diagnostik und Therapie der Arteriosklerose eröffnen.
Similar content being viewed by others
References
Auwerx J, Schoonjans K, Fruchart JC, et al. Transcriptional control of triglyceride metabolism: fibrates change the expression of the LPL and apoC-III genes by activating the nuclear receptor PPAR. Atherosclerosis 1996;124:S29–37.
Averill LE, Meagher RC, Gerrity RD. Enhanced monocyte progenitor cell proliferation in bone marrow of hyperlipemic swine. Am J Pathol 1989;135:369–77.
Basheeruddin K, Rechtoris C, Mazzone T. Transcriptional and post-transcriptional control of apolipoprotein E gene expression in differentiating human monocytes. J Biol Chem 1992;267:1219–24.
Bell FP, Gerrity RG. Evidence for an altered lipid metabolic state in circulating blood monocytes under conditions of hyperlipemia in swine and its implication in arterial lipid metabolism. Arterioscler Thromb 1992;12:155–62.
Bermini F, Didoni G, Bonfadini G, et al. Requirement for mevalonate in acetylated LDL induction of cholesterol esterification in macrophages. Atherosclerosis 1993;104:19–26.
Bhakdi S, Dorweiler B, Kirchmann R, et al. On the pathogenesis of atherosclerosis: enzymatic transformation of human low density lipoprotein to an atherogenic moiety. J Exp Med 1995;182:1959–71.
Bocan TMA, Mazur MJ, Mueller SB, et al. Antiatherosclerotic activity of inhibitors of 3-hydroxy-3-methylglutaryl coenzyme A reductase in cholesterol-fed rabbits: A biochemical and morphological evaluation. Atherosclerosis 1994;111:127–42.
Boisvert WA, Spangenberg J, Curtiss LK. Treatment of severe hypercholesterolemia in apolipoprotein E-deficient mice by bone marrow transplantation. J Clin Invest 1995;96:1118–24.
Casey PJ. Biochemistry of protein prenylation. J Lipid Res 1992;33:1731–40.
Casey PJ. Protein lipidation in cell signalling. Science 1995;268:221–5.
Cerneus DP, Ueffing E, Posthuma G, et al. Detergent insolubility of alkaline phosphatase during biosynthetic transport and endocytosis. J Biol Chem 1993;268:3150–5.
Cianflone KM, Maslowska MH, Sniderman AD. Impaired response of fibroblasts from patients with hyperapobetalipoproteinemia to acylation-stimulating protein. J Clin Invest 1990;85:722–30.
Colli S, Eligini S, Lalli M, et al. Vastatins inhibit tissue factor in cultured human macrophages: A novel mechanism of protection against atherothrombosis. Arterioscler Thromb Vasc Biol 1997;17:265–72.
Corsini A, Raiteri M, Soma MR, et al. Pathogenesis of atherosclerosis and the role of 3-hydroxy-3-methylgutaryl coenzyme A reductase inhibitors. Am J Cardiol 1995;76;Suppl 2:21A-8A.
Corsini A, Raiteri M, Soma MR, et al. Simvastatin but not pravastatin inhibits the proliferation of rat aorta myocytes. Pharmacol Res 1991;23:173–80.
Couturier C, Jahns G, Kazatchkine MD, et al. Membrane molecules which trigger the production of interleukin-1 and tumor necrosis factor-a by lipopolysaccharide-stimulated human monocytes. Eur J Immunol 1992;22:1461–6.
Dallongeville J, Lussier-Cacan S, Davignon J. Modulation of plasma triglyceride levels by apo E phenotype: a meta-analysis. J Lipid Res 1992;33:447–54.
Danielsen EM. A transferrin-like GPI-linked iron-binding protein in detergent-insoluble non-caveolar microdomains at the apical surface of fetal intestinal epithelial cells. Biochemistry 1995;34:1596–605.
Dansky HM, Charlton SA, Harper MM, et al. T and B lymphocytes play a minor role in atherosclerotic plaque formation in the apolipoprotein E-deficient mouse. Proc Natl Acad Sci USA 1997;94:4642–6.
Daugherty A, Puré E, Delfel-Butteiger D, et al. The effects of total lymphocyte deficiency on the extent of atherosclerosis in apolipoprotein E-mice. J Clin Invest 1997;100:1575–80.
De-Maat MP, Pietersma A, Kofflard M, et al. Association of plasma fibrinogen levels with coronary artery disease, smoking and inflammatory markers. Atherosclerosis 1996;121:185–91.
Dentener MA, Bazil V, von Asmuth EJU, et al. Involvement of CD14 in lipopolysaccharide-induced tumor necrosis factor-alpha, IL-6 and IL-8 release by human monocytes and alveolar macrophages. J Immunol 1993;150:2885–91.
Dunon D, Piali L, Imhof BA. To stick or not to stick: the new leukocyte homing paradigm. Curr Opin Cell Biol 1996;8:714–23.
Emeson EE, Shen ML, Bell CG, et al. Inhibition of atherosclerosis in CD4T-cell-ablated and nude (nu/nu) C57BL/6 hyperlipidemic mice. Am J Pathol 1996;149:675–85.
Faruqi RM, Di Corleto PE. Mechanisms of monocyte recruitment and accumulation. Br Heart J 1993;60:S19–29.
Fingerle G, Pforte A, Passlick B, et al. The novel subset of CD14+/CD16+ blood monocytes is expanded in sepsis patients. Blood 1993;82:3170–6.
Forman BM, Tontonoz P, Chen J, et al. 15-Deoxy-Δ12,14-prostaglandin J2 is a ligand for the adipocyte determination factor PPAR gamma. Cell 1995;83:803–12.
Frankenberger M, Sternsdorf T, Pechumer H, et al. Differential cytokine expression in human blood monocyte subpopulations: a PCR analysis. Blood 1996;87:373–7.
Gellman J, Ezekowitz MD, Sarembock IJ, et al. Effect of lovastatin on intimal hyperplasia after balloon angioplasty: A study in an atherosclerotic hypercholesterolemic rabbit. J Am Coll Cardiol 1991;17:251–9.
Geng YJ, Holm J, Nygren S, et al. Expression of macrohage scavenger receptor in atherosclerosis. Relationship between scavenger receptor isforms and the T cell cytokine, interferon-γ. Arterioscler Thromb Vasc Biol 1995;15:1995–2102.
Glomset JA, Gelb MH, Farnsworth CC. Prenyl proteins in eukaryotic cells: A new type of membrane anchor. TIBS 1990;15:139–42.
Goldstein JL, Brown MS. Regulation of the mevalonate pathway. nature 1990;343:425–30.
Grayston JT, Kuo CC, Campbell LA, et al. Chlamydia pneumoniae, strain TWAR and atherosclerosis. Eur Heart J 1993;14:Suppl K:66–71.
Grewal IS, Flavell RA. A central role of CD40 ligand in the regulation of CD4+T-cell responses. Immunol Today 1996;17:410–4.
Grunler J, Ericsson J, Dallner G. Branch-point reactions in the biosynthesis of choleserol, dolichol, ubiquinone and prenylated proteins. Biochim Biophys Acta 1994;1212:259–77.
Gupta S, Pablo AM, Jiang XC, et al. IFN-γ potentiates atherosclerosis in apo E knock-out mice. J Clin Invest 1997;99:2752–61.
Hanada K, Nishijima M, Akamatsu Y, et al. Both sphingolipids and cholesterol participate in the detergent-insolubility of alkali ne phosphatase, a glycosyl-phosphatidylinositol anchored protein in mammalian cell. J Biol Chem 1995;270:6254–60.
Hannan LA, Lisanti M, Rodriguez-Boulan E, et al. Correctly sorted molecules of a GPI-anchored protein are clustered and immobile when they arrive at the apical surface of MDCK cell. J Cell Biol 1993;120:353–8.
Hansson GK, Holm J, Jonasson L. Detection of activated T lymphocytes in the human atherosclerotic plaque. Am J Pathol 1989;135:169–75.
Hansson GK, Libby P. In: Fuster V, Ross R, Topol EJ, eds. Atherosclerosis and coronary artery disease, Vol. 1. Philadelphia: Lippincott-Raven, 1996:557–68.
Hansson GK. Cell-mediated immunity in atherosclerosis. Curr Opin Lipidol 1997;8:301–11.
Henney AM, Wakeley PR, Davies MJ, et al. Localization of stromelysin gene expression in atherosclerotic plaques by in situ hybridization. Proc Natl Acad Sci USA 1991;88:8154–8.
Hertz R, Berman I, Bar-Tana J. Transcriptional activation by amphipathic carboxylic peroxisomal proliferators is induced by the free acid rather than the acyl-CoA derivative. Eur J Biochem 1994;221:611–5.
Hughes DA, Townsend PJ, Haslam PL. Enhancement of the antigen-presenting function of monocytes by cholesterol: possible relevance to inflammatory mechanisms in extrinsic allergic alveolitis and atherosclerosis. Clin Exp Immunol 1992;87:279–86.
Ip JH, Fuster V, Badimon L, et al. Syndromes of accelerated atherosclerosis: role of vascular injury and smooth muscle cell proliferation. J Am Coll Cardiol 1990;15:1667–87.
Jackson LA, Campbell LA, Schmidt RA, et al. Specificity of detection of Chlamydia pneumoniae in cardiovascular atheroma: evaluation of the innocent bystander hypothesis. Am J Pathol 1997;150:1785–90.
Jiang C, Ting AT, Seed B. PPAR-γ agonists inhibit production of monocyte inflammatory cytokines. Nature 1998;391:82–6.
Jonasson L, Holm J, Skalli O, et al. Expression of class II transplantation antigen on vascular smooth muscle cells in human atherosclerosis. J Clin Invest 1985;76:125–31.
Kannel WB, Anderson K, Wilson PWF. White blood cell count and cadiovascular disease: insights from the Framingham study. JAMA 1992;267:1253–6.
Kiener PA, Rankin BM, Burkhardt AL, et al. Cross-linking of Fc gamma receptor I (FcγRI) and receptor II (FcγRII) on monocytic cells activates a signal transduction pathway common to both Fc receptors that involves the stimulation of p72 Syk protein tyrosine kinase. J Biol Chem 1993;268:2442–8.
Kim DH, Iijima H, Goto K, et al. Human apolipoprotein Ereceptor 2. A novel lipoprotein receptor of the low density lipoprotein receptor family predominantly expressed in brain. J Biol Chem 1996;271:8373–80.
Kishikawa H, Shimokama T, Watanabe T. Localization of T lymphocytes and macrophages expressing IL-1, IL-2 receptor, IL-6 and TNF in human aortic intima. Role of cell mediated immunity in human atherogenesis. Virchows Arch pathol Anat 1993;423:433–42.
Kobashigawa JA, Katznelson S, Laks H, et al. Effect of pravastatin on outcomes after cardiac transplantation. N Engl J Med 1995;333:621–7.
Kuo CC, Gown AM, Benditt EP, et al. Detection of Chlamydia pneumoniae in aortic lesions of atherosclerosis by immunocytochemical stain. Arterioscler Thromb 1993;13:1501–4
Lehmann JM, Moore LB, Smith-Oliver TA, et al. An antidiabetic thiazolidinedione is a high affinity ligand for peroxisome proliferator-activated receptor gamma (PPAR-γ). J Biol Chem 1995;270:12953–6.
Linton MF, Atkinson JB, Fazio S. Prevention of atherosclerosis in apoliprotein E-deficient mice by bone marrow transplantation. Science 1995;267:1034–7.
Locher C, Vanham G, Kestens L, et al. Expression patterns of Fc gamma receptors, HLA-DR and selected adhesion molecules on monocytes from normal and HIV-infected individuals. Clin Exp Immunol 1994;98:115–22.
Mach F, Schonbeck U, Sukhova GK, et al. Functional CD40 ligand is expressed on human vascular endothelial cells, smooth muscle cells and macrophages-implications for CD40-CD40 ligand signaling in atherosclerosis. Proc Natl Acad Sci USA 1997;94:1931–6.
Mahley RW. Apolipoprotein E: cholesterol transport protein with expanding role in cell biology. Science 1988;240:622–30.
Maltese WA. Posttranslational modification of proteins by isoprenoids in mammalian cells. FASEB J 1990;4:3319–28.
Munro JM, van der Walt JD, Munro CS, et al. An immunohistochemical analysis of human aortic fatly streaks. Hum Pathol 1987;18:375–80.
Murakami T, Yamada N. Modification of macrophage function and effects on atherosclerosis. Curr Opin Lipidol 1996;7:320–3.
Nieto FJ, Adam E, Sorlie P, et al. Cohort study of cytomegalovirus infection as a risk factor for carotid intimal-medical thickening, a measure of subclinical atherosclerosis. Circulation 1996;94:922–7.
Nockher WA, Bergmann L, Scherberich JE. Increased soluble CD14 serum levels and altered CD14 expression of peripheral blood monocytes in HIV-infected patients. Clin Exp Immunol 1994;98:369–74.
O’Brien KD, Allen MD, McDonald TO, et al. Vascular adhesion molecule-1 is expressed in human coronary atherosclerotic plaques. J Clin Invest 1993;92:945–51.
Passlick B, Flieger D, Ziegler-Heitbrock HWL. Identification and characterization of a novel monocyte subpopulation in human peripheral blood. Blood 1989;74:2527–34.
Passlick B, Pforte A. The novel subset of CD14+/CD16+ blood monocytes exhibits features of tissue macrophages. Eur J Immunol 1993;23:2053–8.
Peng X, Kasran A, Warmerdam PAM, et al. Accessory signaling by CD40 for T cell activation: induction of Th1 and Th2 cytokines and synergy with interleukin-12 for interferon-gamma production. Eur J Immunol 1996;26:1621–7.
Peters JH, Gieseler R, Thiele B, et al. Dendritic cells: from ontogenetic orphans to myelomonocytic descendants. Immunol Today 1996;17:273–8.
Plump AS, Breslow JL. Apolipoprotein E and the apolipoprotein E-deficient mouse. Annu Rev Nutr 1995;15:495–518.
Plump AS, Smith JD, Hayek T, et al. Severe hyperchoiesterolemia and atherosclerosis in apolipoprotein E-deficient mice created by homologous recombination in ES cells. Cell 1992;71:343–53.
Poston PN, Haskard DO, Coucher JR, et al. Expression of intercellular adhesion molecule-1 in atherosclerotic plaques. Am J Pathol 1992;140:665–73.
Puolakkainen M, Kuo CC, Shor A, et al. Serological response to Chlamydia pneumoniae in adults with coronary arterial fatty streaks and fibrolipid plaques. J Clin Microbiol 1993;31:2212–4.
Rankin BM, Yocum SA, Mittler RS, et al. Stimulation of tyrosine phosphorylation and calcium mobilization by Fcγ receptor crosslinking. Regulation by the phosphotyrosine phosphatase CD45. J Immunol 1993;150:605–16.
Ricote M, Li AC, Willson TM, et al. The peroxisome proliferatoractivated receptor-γ-is a negative regulator of macrophage activation. Nature 1998;391:79–82.
Ridker PM, Cushman M, Stampfer MJ, et al. Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. N Engl J Med 1997;336:973–9.
Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature 1993;362:801–9.
Rothe G, Gabriel H, Kovacs E, et al. Peripheral blood mononuclear phagocyte subpopulations as cellular markers in hypercholesterolemia. Arterioscler Thromb Vasc Biol 1996;16:1437–47.
Rothe G, Herr AS, Stöhr J, et al. A more mature phenotype of blood mononuclear phagocytes is induced by fluvastatin treatment in hypercholesterolemia. Atherosclerosis (in press).
Rothe G, Stöhr J, Fehringer P, et al. Altered mononuclear phagocyte differentiation associated with genetic defects of the lysosoma acid lipase. Atherosclerosis 1997;130:215–21.
Saikku P. Chlamydia pneumoniae infection as a risk factor in acute myocardial infarction. Eur Heart J 1993;14:Suppl K:62–5.
Sakai M, Koboti S, Matsumura T, et al. HMG-CoA reductase inhibitors suppress macrophage growth induced by oxidized low density lipoprotein. Atherosclerosis 1997;133:51–9.
Saleh MN, Goldman J, Lo Buglio AF, et al. CD16+monocytes in patients with cancer: spontaneous elevation and pharmacologic induction by recombinant human macrophage colony-stimulating factor. Blood 1995;85:2910–7.
Sargiacomo M, Sudol M, Tang Z, et al. Signal transducing molecules and GPI-linked proteins form a caveolin-rich insoluble complex in MDCK cells. J Cell Biol 1993;122:789–807.
Saunders AM, Strittmatter WJ, Schmechel D, et al. Association of apolipoprotein E allele e4 with late-onset familial and sporadic Alzheimer’s disease. Neurology (NY) 1993;43:1467–72.
Schmid I, Baldwin GC, Jacobs EL, et al. Alterations in phenotype and cell-surface antigen expression levels of human monocytes: differential response to in vivo administration of rhM-CSF or rhGM-CSF. Cytometry 1995;22:103–10.
Schoonjans K, Staels B, Auwerx J. Role of the peroxisome proliferator-activated receptor (PPAR) in mediating the effects of fibrates and fatty acids on gene expression. J Lipid Res 1996;37:907–25.
Schütt C, Ringel B, Nausch M, et al. Human monocyte activation induced by an anti-CD14 monoclonal antibody. FEBS Lett 1988;19:321–8.
Selwyn AP, Kinlay S, Creager M, et al. Cell dysfunction in atherosclerosis and the ischemic manifestations of Coronary artery disease. Am J Cardiol 1997;79:17–23.
Shimokama T, Haraoka S, Watanabe T. Immunohistochemical and ultrastructural demonstration of the lymphocyte-macrophage interaction in human aortic intima. Modern Pathol 1991;4:101–7.
Shimokama T, Haraoka S, Watanabe T. Morphological fate and sequelae of human atherosclerosis: evaluation of immune mechanisms in atherogenesis through immunohistologic and ultrastructural analysis. Pathol Int 1995;45:801–14.
Simons K, Ikonen E. Functional rafts in cell membranes. Nature 1997;387:569–72.
Sinensky M, Lutz RJ. The prenylation of proteins. Bioessays 1992;14:25–31.
Skibbens JE, Roth MG, Matlin KS. Differential extractibility of influenza virus hemaglutinin during intracellular transport in polarized epithelial cells and nonpolar fibroblasts. J Cell Biol 1989;108:821–32.
Smith JD, Trogan E, Ginsberg M, et al. Decreased atherosclerosis in mice deficient in both macrophage colony-stimulating factor (op) and apolipoportein E. Proc Natl Acad Sci USA 1995;92:8264–8.
Soma MR, Donetti E, Parolini C, et al. HMG-CoA reductase inhibitors: In vivo effects on carotid intimal thickening in normocholesterolemic rabbits. Arterioscler Thromb 1993;13:571–8.
Stemme S, Faber B, Holm J, et al. T lymphocytes from human atherosclerotic plaques recognize oxidized LDL. Proc Natl Acad Sci USA 1995;92:3893–7.
Stemme S, Holm J, Hansson GK. T lymphocytes in human atherosclerotic plaques are memory cells expressing CD45RO and the integrin VLA-1. Arterioscler Thromb 1992;12:206–11.
Stemme S, Rymo L, Hansson GK. Polyclonal origin of T lymphocytes in human atherosclerotic plaque. Lab Invest 1991;65:654–60.
Stöhr J, Schindler G, Rothe G, et al. Enhanced upregulation of the Fcγ receptor IIIa (CD16a) during in vitro differentiation of Apo E4/4 monocytes. Arterioscler Thromb Vasc Biol (in press).
Stragliotto E, Camera M, Postiglione A, et al. Functionally abnormal monocytes in hypercholesterolemia. Arterioscler Thromb 1993;13:944–50.
Strittmatter WJ, Saunders AM, Schmechel D, et al. Apolipoprotein E: high-avidity binding to b-amyloid and increased frequency of type 4 allele in late-onset familial Alzheimer disease. Proc Natl Acad Sci USA 1993;90:1977–81.
Taraboulos A, Scott M, Semenov A, et al. Cholesterol depletion and modification of COOH-terminal targeting sequence of the prion protein inhibit formation of the scrapie isoform. J Cell Biol 1995;129:121–32.
Utermann G, Pruin N, Steinmetz A. Polymorphism of apolipoprotein E. III. Effect of a single polymorphic gene locus on plasma lipid levels in man. Clin Genet 1979;15:63–72.
van der Wal AC, Das PK, van de Berg DB, et al. Atherosclerotic lesions in humans. In situ immunophenotypic analysis suggesting an immune mediated response. Lab Invest 1989;61:166–70.
Walladius G. Fatty acid incorporation into human adipose tissue in hypertriglyceridemia. Methodological, clinical and experimental studies. Acta Med Scand 1976;591:1–47.
Watanabe T, Haraoka S, Shimokama T. Inflammatory and immunological nature of atherosclerosis. Int J Cardiol 1996;54:S1–60.
Weiss A, Littman DR. Signal transduction by lymphocyte antigen receptors. Cell 1994;76:263–74.
Wick G, Schett G, Amberger A, et al. Is atherosclerosis an immunologically mediated disease? Immunol Today 1995;16: 27–33.
Wright SD, Ramos RA, Tobias PS, et al. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science 1990;249:1431–3.
Xu Q, Wick G. The role of heat shock proteins in protection and pathophysiology of the arterial wall. Mol Med Today 1996;Sept:372–9.
Zhu BQ, Sievers RE, Sun YP, et al. Effect of lovastatin on suppression and regression of atherosclerosis in lipid-fed rabbits. J Cardiovasc Pharmacol 1992;19:246–55.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Schmitz, G., Herr, A.S. & Rothe, G. T-lymphocytes and monocytes in atherogenesis. Herz 23, 168–177 (1998). https://doi.org/10.1007/BF03044602
Issue Date:
DOI: https://doi.org/10.1007/BF03044602