Summary
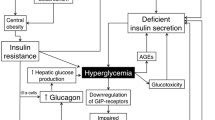
Prevention and treatment of type 2 diabetes mellitus (T2DM) and the metabolic syndrome represent a major clinical challenge, because effective strategies such as fat restriction and exercise are difficult to implement into diabetes treatment. Based on the increasing knowledge on the pathogenesis of T2DM, new therapeutic approaches are currently under investigation. Potential targets of new therapeutic approaches include: (i) Inhibition of hepatic glucose production, (ii) stimulation of glucose-dependent insulin secretion, (iii) enhancement of insulin signal transduction, and (iv) reduction of body fat mass. Agonists of glucagon-like-peptide 1 (GLP-1) and antagonists of dipeptidylpeptidase IV, which inactivates GLP-1, stimulate glucose-dependent insulin secretion, improve hyperglycemia and are already tested in clinical trials. In humans, glucagon antagonists and an amylin analogue reduce glucagon-dependent glucose production. The glucose-lowering effect of current modulators of lipid oxidation is not pronounced and their use could be limited by side effects. In addition to clinically approved thiazolidendiones, new agonists of the peroxisome proliferator activator receptor γ (PPARγ) as well as combined PPAR α/γ agonists are developed at present. The direct modulation of insulin signal transduction is still limited to experimental studies.
Zusammenfassung
Die Prävention und Behandlung des Typ-2-Diabetes mellitus (T2DM) und des metabolischen Syndroms stellen eine große klinische Herausforderung dar, da wirksame therapeutische Strategien wie fettarme Diät und körperliche Bewegung derzeit nur unzureichend umgesetzt werden. Aufgrund des zunehmenden Wissens über die Pathogenese des T2DM werden neue Therapieansätze geprüft. Angriffspunkte solcher Therapieformen sind (i) die Hemmung der Glukose-produktion der Leber, (ii) die Stimulation der Glukoseabhängigen Insulinsekretion, (iii) die Verbesserung der Insulin-Signaltransduktion und (iv) die Reduktion der Fettgewebsmasse. Agonisten des Glukagon-like-peptide (GLP-1) und Antagonisten der Dipeptidylpeptidase-IV, die GLP-1 abbauen, stimulieren die Glukose-abhängige Insulinsekretion, verbessern so die Hyperglykämie deutlich und sind bereits in klinischer Prüfung. Beim Menschen reduzieren Glukagon-Antagonisten sowie ein Amylin-Analog die Glukagon-abhängige Glukoseproduktion. Die Blutzucker-senkende Wirkung vorhandener Modulatoren der Lipidoxidation ist nicht ausgeprägt und könnte durch Nebenwirkungen limitiert sein. Neben bereits zugelassenen Thiazolidendion-Präparaten werden weitere Agonisten des peroxisome proliferator activator receptors γ (PPARγ) sowie kombinierte PPARα/γ-Agonisten untersucht. Die direkte Modulation der Insulin-Signaltransduktion ist derzeit noch auf experimentelle Untersuchungen beschränkt.
Similar content being viewed by others
Literatur
Harris MI, Flegal KM, Cowie CC, Eberhardt MS, Goldstein DE, Little RR, Wiedmeyer HM, Byrd-Holt DD, (1998) Prevalence of diabetes, impaired fasting glucose, and impaired glucose tolerance in U.S. adults. The Third National Health and Nutrition Examination Survey, 1988–1994. Diabetes Care 21: 518–524
Rosenbloom AL, Joe JR, Young RS, Winter WE (1999) Emerging epidemic of type 2 diabetes in youth. Diabetes Care 22: 345–354
Reaven GM (1992) The role of insulin resistance and hyperinsulinemia in coronary heart disease. Metabolism 41: 16–19
DeFronzo RA, Simonson D, Ferrannini E (1982) Hepatic and peripheral insulin resistance: a common feature of type 2 (non-insulin-dependent) and type 1 (insulin-dependent) diabetes mellitus. Diabetologia 23: 313–319.
Laws A, Reaven GM (1992) Evidence for an independent relationship between insulin resistance and fasting plasma HDL-cholesterol, triglyceride and insulin concentrations. J Intern Med 231: 25–30
UK Prospective Diabetes Study Group (UKPDS) (1998) Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) Lancet 352: 837–853
UK Prospective Diabetes Study Group (1998) Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ 317: 703–713
Gaede P, Vedel P, Larsen N, Jensen GV, Parving HH, Pedersen O (2003) Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N Engl J Med 348: 383–393
Inzucchi SE (2002) Oral antihyperglycemic therapy for type 2 diabetes: scientific review. JAMA 287: 360–372
Schernthaner G (2003) Fortschritte in der Prävention des Typ-2-Diabetes. Wien Klin Wochenschr 115: 745–757
Ekberg K, Landau BR, Wajngot A, Chandramouli V, Efendic S, Brunengraber H, Wahren J (1999) Contributions by kidney and liver to glucose production in the postabsorptive state and after 60 h of fasting. Diabetes 48: 292–298
Pagliassotti MJ, Cherrington AD (1992) Regulation of net hepatic glucose uptake in vivo. Annu Rev Physiol 54: 847–860
Consoli A (1992) Role of liver in pathophysiology of NIDDM. Diabetes Care 15: 430–441
Radziuk J, Pye S (2002) Quantitation of basal endogenous glucose production in type II diabetes: importance of the volume of distribution. Diabetologia 45: 1053–1084
Hundal RS, Krssak M, Dufour S, Laurent D, Lebon V, Chandramouli V, Inzucchi SE, Schumann WC, Petersen KF, Landau BR, Shulman GI (2000) Mechanism by which metformin reduces glucose production in type 2 diabetes. Diabetes 49: 2063–2069
Magnusson I, Rothman DL, Katz LD, Shulman RG, Shulman GI (1992) Increased rate of gluconeogenesis in type II diabetes mellitus. A13C nuclear magnetic resonance study. J Clin Invest 90: 1323–1327
Inzucchi SE, Maggs DG, Spollett GR, Page SL, Rife FS, Walton V, Shulman GI (1998) Efficacy and metabolic effects of metformin and troglitazone in type II diabetes mellitus. N Engl J Med 338: 867–872
Roden M, Perseghin G, Petersen KF, Hwang JH, Cline GW, Gerow K, Rothman DL, Shulman GI (1996) The roles of insulin and glucagon in the regulation of hepatic glycogen synthesis and turnover in humans. J Clin Invest 97: 642–648
Unger RH (1971) Glucagon physiology and pathophysiology. N Engl J Med 285: 443–449
Cherrington AD, Williams PE, Shulman GI, Lacy WW (1981) Differential time course of glucagon’s effect on glycogenolysis and gluconeogenesis in the conscious dog. Diabetes 30: 180–187
Shah P, Vella A, Basu A, Schwenk WF, Rizza RA (2000) Lack of suppression of glucagon contributes to postprandial hyperglycemia in subjects with type 2 diabetes mellitus. J Clin Endocrinol Metab 85: 4053–4059
Connell R (1999) Glucagon antagonists for the treatment of type 2 diabetes. Exp Opin Ther Patents 9: 701–709
Unson CG, Andreu D, Gurzenda EM, Merrifield RB (1987) Synthetic peptide antagonists of glucagon. Proc Natl Acad Sci USA 84: 4083–4087
Brand CL, Rolin B, Jorgensen PN, Svendsen I, Kristensen JS, Holst JJ (1994) Immunoneutralization of endogenous glucagon with monoclonal glucagon antibody normalizes hyperglycaemia in moderately streptozotocin-diabetic rats. Diabetologia 37: 985–993
Petersen KF, Sullivan JT (2001) Effects of a novel glucagon receptor antagonist (Bay 27-9955) on glucagon-stimulated glucose production in humans. Diabetologia 44: 2018–2024
Young A, Denaro M (1998) Roles of amylin in diabetes and in regulation of nutrient load. Nutrition 14: 524–527
Nyholm B, Brock B, Orskov L, Schmitz O (2001) Amylin receptor agonists: a novel pharmacological approach in the management of insulin-treated diabetes mellitus. Expert Opin Investig Drugs 10: 1641–1652
Kahn SE, Andrikopoulos S, Verchere CB (1999) Islet amyloid: a long-recognized but underappreciated pathological feature of type 2 diabetes. Diabetes 48: 241–253
Hollander PA, Levy P, Fineman MS, Maggs DG, Shen LZ, Strobel SA, Weyer C, Kolterman OG (2003) Pramlintide as an adjunct to insulin therapy improves long-term glycemic and weight control in patients with type 2 diabetes: a 1-year randomized controlled trial. Diabetes Care 26: 784–790
Fineman M, Weyer C, Maggs DG, Strobel S, Kolterman OG (2002) The human amylin analog, pramlintide, reduces postprandial hyperglucagonemia in patients with type 2 diabetes mellitus. Horm Metab Res 34: 504–508
Thompson RG, Gottlieb A, Organ K, Koda J, Kisicki J, Kolternan OG (1997) Pramlintide: a human amylin analogue reduced postprandial plasma glucose, insulin, and C-peptide concentrations in patients with type 2 diabetes. Diabet Med 14: 547–555
Weyer C, Maggs DG, Young AA, Kolterman OG (2001) Amylin replacement with pramlintide as an adjunct to insulin therapy in type 1 and type 2 diabetes mellitus: a physiological approach toward improved metabolic control. Curr Pharm Des 7: 1353–1373
Ratner RE, Want LL, Fineman MS, Velte MJ, Ruggles JA, Gottlieb A, Weyer C, Kolterman OG (2002) Adjunctive therapy with the amylin analogue pramlintide leads to a combined improvement in glycemic and weight control in insulin-treated subjects with type 2 diabetes. Diabetes Technol Ther 4: 51–61
Barlocco D (2001) Parmlintide (Amylin). Curr Opin Investig Drugs 2: 1575–1581
Treadway JL, Mendys P, Hoover DJ (2001) Glycogen phosphorylase inhibitors for treatment of type 2 diabetes mellitus. Expert Opin Investig Drugs 10: 439–454
Martin WH, Hoover DJ, Armento SJ, Stock IA, McPherson RK, Danley DE, Stevenson RW, Barrett EJ, Treadway JL (1998) Discovery of a human liver glycogen phosphorylase inhibitor that lowers blood glucose in vivo. Proc Natl Acad Sci USA 95: 1776–1781
Goto M, Zeller WP, Hurley RM (1991) Effects of cytarabine on glucoregulation in suckling rats. Res Commun Chem Pathol Pharmacol 71: 189–194
Zhang BB, Moller DE (2000) New approaches in the treatment of type 2 diabetes. Curr Opin Chem Biol 4: 461–467
Parker JC, Van Volkenburg MA, Levy CB, Martin WH, Burk SH, Kwon Y, Giragossian C, Gant TG, Carpino PA, McPherson RK, Vestergaard P, Treadway JL (1998) Plasma glucose levels are reduced in rats and mice treated with an inhibitor of glucose-6-phosphate translocase. Diabetes 47: 1630–1636
Andrews RC, Rooyackers O, Walker BR (2003) Effects of the 11 beta-hydroxysteroid dehydrogenase inhibitor carbenoxolone on insulin sensitivity in men with type 2 diabetes. J Clin Endocrinol Metab 88: 285–291
Keilson L, Mather S, Walter YH, Subramanian S, McLeod JF (2000) Synergistic effects of nateglinide and meal administration on insulin secretion in patients with type 2 diabetes mellitus. J Clin Endocrinol Metab 85: 1081–1086
Wolffenbuttel BH, Landgraf R (1999) A 1-year multicenter randomized double-blind comparison of repaglinide and glyburide for the treatment of type 2 diabetes. Dutch and German Repaglinide Study Group. Diabetes Care 22: 463–467
Marbury T, Huang WC, Strange P, Lebovitz H (1999) Repalinide versus glyburide: a one-year comparison trial. Diabetes Res Clin Pract 43: 155–166
Landgraf R, Bilo HJ, Muller PG (1999) A comparison of repaglinide and glibenclamide in the treatment of type 2 diabetic patients previously treated with sulphonylureas. Eur J Clin Pharmacol 55: 165–171
Edwards CM, Todd JF, Mahmoudi, M, Wang Z, Wang RM, Ghatei MA, Bloom SR (1999) Glucagon-like peptide 1 has a physiological role in the control of postprandial glucose in humans: studies with the antagonist exendin 9–39 Diabetes 48: 86–93
Drucker DJ (2001) Minireview: the glucagon-like peptides. Endocrinology 142: 521–527
Moller DE (2001) New drug targets for type 2 diabetes and the metabolic syndrome. Nature 414: 821–827
Miyawaki K, Yamada Y, Yano H, Niwa H, Ban N, Ihara Y, Kubota A, Fujimoto S, Kajikawa M, Kuroe A, Tsuda K, Hashimoto H, Yamashita T, Jomori T, Tashiro F, Miyazaki J, Seino Y (1999) Glucose intolerance caused by a defect in the entero-insular axis: a study in gastric inhibitory polypeptide receptor knockout mice. Proc Natl Acad Sci USA 96: 14843–14847
Farilla L, Hui H, Bertolotto C, Kang E, Bulotta A, Di Mario U, Perfetti R (2002) Glucagon-like peptide-1 promotes islet cell growth and inhibits apoptosis in Zucker diabetic rats. Endocrinology 143: 4397–4408
Raufman JP, Singh L, Singh G, Eng J (1992) Truncated glucagon-like peptide-1 interacts with exendin receptors on dispersed acini from guinea pig pancreas. Identification of a mammalian analogue of the reptilian peptide exendin-4. J Biol Chem 267: 21432–21437
Goke R, Fehmann HC, Linn T, Schmidt H, Krause M, Eng J, Goke B (1993) Exendin-4 is a high potency agonist and truncated exendin-(9–39)-amide an antagonist at the glucagon-like peptide 1-(7-36)-amide receptor of insulin-secreting beta-cells, J Biol Chem 268; 19650–19655
Xu G, Stoffers DA, Habener JF, Bonner-Weir S (1999) Exendin-4 stimulates both beta-cell replication and neogenesis, resulting in increased beta-cell mass and improved glucose tolerance in diabetic rats. Diabetes 48: 2270–2276
Szayna M, Doyle ME, Betkey JA, Holloway HW, Spencer RG, Greig NH, Egan JM (2000) Exendin-4 decelerates food intake, weight gain, and fat deposition in Zucker rats. Endocrinology 141: 1936–1941
Edwards CM, Stanley SA, Davis R, Brynes AE, Frost GS, Seal LJ, Ghatei MA, Bloom SR (2001) Exendin-4 reduces fasting and postprandial glucose and decreases energy intake in healthy volunteers. Am J Physiol Endocrinol Metab 281: E155–161
Egan JM, Meneilly GS, Elahi D (2003) Effects of 1-mobolus subcutaneous administration of exendin-4 in type 2 diabetes. Am J Physiol Endocrinol Metab 284: E1072–1079
Rolin B, Larsen MO, Gotfredsen CF, Deacon CF, Carr RD, Wilken M, Knudsen LB (2002) The long-acting GLP-1 derivative NN2211 ameliorates glycemia and increases beta-cell mass in diabetic mice. Am J Physiol Endocrinol Metab 283: E745–752
Larsen PJ, Fledelius C, Knudsen LB, Tang-Christensen M (2001) Systemic administration of the long-acting GLP-1 derivative NN2211 induces lasting and reversible weight loss in both normal and obese rats. Diabetes 50: 2530–2539
Juhl CB, Hollingdal M, Sturis J, Jakobsen G, Agerso H, Veldhuis J, Porksen N, Schmitz O (2002) Bedtime administration of NN2211, a long-acting GLP-1 derivative, substantially reduces fasting and postprandial glycemia in type 2 diabetes. Diabetes 51: 424–429
Marguet D, Baggio L, Kobayashi T, Bernard AM, Pierres M, Nielsen PF, Ribel U, Watanabe T, Drucker DJ, Wagtmann N (2000) Ennanced insulin secretion and improved glucose tolerance in mice lacking CD26. Proc Natl Acad Sci USA 97: 6874–6879
Pospisilik JA, Stafford SG, Demuth HU, McIntosh CH, Pederson RA (2002) Long-term treatment with dipeptidyl peptidase IV inhibitor improves hepatic and peripheral insulin sensitivity in the VDF Zucker rat: a euglycemichyperin sulinemic clamp study. Diabetes 51: 2677–2683
Reimer MK, Hoist JJ, Ahren B (2002) Long-term inhibition of dipeptidyl peptidase IV improves glucose tolerance and preserves islet function in mice. Eur J Endocrinol 146: 717–727
Sudre B, Broqua P, White RB, Ashworth D, Evans DM, Haigh R, Junien JL, Aubert ML (2002) Chronic inhibition of circulating dipeptidyl peptidase IV by FE 999011 delays the occurrence of diabetes in male zucker diabetic fatty rats. Diabetes 51: 1461–1469
Ahren B, Simonsson E, Larsson H, Landin-Olsson M, Torgeirsson H, Jansson PA, Sandqvist M, Bavenholm P, Efendic S, Eriksson JW, Dickinson S, Holmes D (2002) Inhibition of dipeptidyl peptidase IV improves metabolic control over a 4-week study period in type 2 diabetes. Diabetes Care 25: 869–875
Saltiel AR, Kahn CR (2001) Insulin signalling and the regulation of glucose and lipid metabolism. Nature 414: 799–806
Zhang B, Salituro G, Szalkowski D, Li Z, Zhang Y, Royo I, Vilella D, Diez MT, Pelaez F, Ruby C, Kendall RL, Mao X, Griffin P, Calaycay J, Zierath JR, Heck IV, Smith RG, Moller DE (1999) Discovery of a small molecule insulin mimetic with antidiabetic activity in mice. Science 284: 974–977
Manchem VP, Goldfine ID, Kohanski RA, Cristobal CP, Lum RT, Schow SR, Shi S, Spevak WR, Laborde E, Toavs DK, Villar HO, Wick MM, Kozlowski MR (2001) A novel small molecule that directly sensitizes the insulin receptor in vitro and in vivo. Diabetes 50: 824–830
Ryan GJ, Wanko NS, Redman AR, Cook CB (2003) Chromium as adjunctive treatment for type 2 diabetes. Ann Pharmacother 37: 876–885
Anderson RA (1998) Chromium, glucose intolerance and diabetes. J Am Coll Nutr 17: 548–555
Althuis MD, Jordan NE, Ludington EA, Wittes JT (2002) Glucose and insulin responses to dietary chromium supplements: a meta-analysis. Am J Clin Nutr 76: 148–155
Meyerovitch J, Rothenberg P, Shechter Y, Bonner-Wier S, Kahn CR (1991) Vanadate normalizes hypergly cemia in two mouse models of non-insulin-dependent diabetes mellitus. J Clin Invest 87: 1286–1294
Halberstam M, Cohen N, Shlimovich P, Rossetti L, Shamoon H (1996) Oral vanadyl sulfate improves insulin sensitivity in NIDDM but not in obese nondiabetic subjects. Diabetes 45: 659–666
Srivastava AK (2000) Anti-diabetic and toxic effects of vanadium compounds. Mol Cell Biochem 206: 177–182
Cohen N, Halberstam M, Shlimovich P, Chang CJ, Shamoon H, Rossetti L (1995) Oral vanadyl sulfate improves hepatic and peripheral insulin sensitivity in patients with non-insulin-dependent diabetes mellitus. J Clin Invest 95: 2501–2509
Elchebly M, Payette P, Michaliszyn E, Cromlish W, Collins S, Loy AL, Normandin D, Cheng A, Himms-Hagen J, Chan CC, Ramachandran C, Gresser MJ, Tremblay ML, Kennedy BP (1999) Increased insulin sensitivity and obesity resistance in mice lacking the protein tyrosine phosphatase-1B gene. Science 283: 1544–1548
Bush EN, Shapiro R, Clampit J, Kennedy M, Mika A, Jacobson P, Trevillyan J, Jirousek M, Zinker B (2001) Treatment of Zucker diabetic fatty (ZDF) rats with antisense oligonucleotide to phosphotyrosine phosphatase-1B for 5 weeks halts development of diabetes. Diabetes 50: A81
Weston CR, Davis RJ (2001) Signal transduction: signaling specificity — a complex affair. Science 292: 2439–2440
Nikoulina SE, Ciaraldi TP, Mudaliar S, Mohideen P, Carter L, Henry RR (2000) Potential role of glycogen synthase kinase-3 in skeletal muscle insulin resistance of type 2 diabetes. Diabetes 49: 263–271
Cline GW, Johnson K, Regittnig W, Perret P, Tozzo E, Xiao L, Damico C, Shulman GI (2002) Effects of a novel glycogen synthase kinase-3 inhibitor on insulin-stimulated glucose metabolism in Zucker diabetic fatty (fa/fa) rats. Diabetes 51: 2903–2910
Muller G (2000) The molecular mechanism of the insulinmimetic/sensitizing activity of the antidiabetic sulfonylurea drug amaryl. Mol Med 6: 907–933
Muller G (2002) Dynamics of plasma membrane microdomains and cross-talk to the insulin signalling cascade. FEBS Lett 531: 81–87
Overkamp D, Volk A, Maerker E, Heide PE, Wahl HG, Rett K, Haring HU (2002) Acute effect of glimepiride on insulin-stimulated glucose metabolism in glucose-tolerant insulin-resistant offspring of patients with type 2 diabetes. Diabetes Care 25: 2065–2073
Marette E (2002) Mediators of cytokine-induced insulin resistance in obesity and other inflammatory settings. Curr Opin Clin Nutr Metab Care 5: 377–383
Yuan M, Konstantopoulos N, Lee J, Hansen L, Li ZW, Karin M, Shoelson SE (2001) Reversal of obesity- and diet-induced insulin resistance with salicylates or targeted disruption of Ikkbeta. Science 293: 1673–1677
Kim JK, Kim YJ, Fillmore JJ, Chen Y, Moore I, Lee J, Yuan M, Li ZW, Karin M, Perret P, Shoelson SE, Shulman GI (2001) Prevention of fat-induced insulin resistance by salicylate. J Clin Invest 108: 437–446
Felber JP, Ferrannini E, Golay A, Meyer HU, Theibaud D, Curchod B, Maeder E, Jequier E, DeFronzo RA (1987) Role of lipid oxidation in pathogenesis of insulin resistance of obesity and type II diabetes. Diabetes 36: 1341–1350
Reaven GM, Holenbeck C, Jeng CY, Wu MS, Chen YD (1988) Measurement of plasma glucose, free fatty acid, lactate, and insulin for 24 h in patients with NIDDM. Diabetes 37: 1020–1024
Roden M, Krssak M, Stingl H, Gruber S, Hofer A, Furnsinn C, Moser E, Waldhausl W (1999) Rapid impairment of skeletal muscle glucose transport/phosphorylation by free fatty acids in humans. Diabetes 48: 358–364
Roden M, Stingl H, Chandramouli V, Schumann WC, Hofer A, Landau BR, Nowotny P, Waldhausl W, Shulman GI (2000) Effects of free fatty acid elevation on postabsorptive endogenous glucose production and gluconeogenesis in humans. Diabetes 49: 701–707
Stingl H, Krssak M, Krebs M, Bischof MG, Nowotny P, Furnsinn C, Shulman GI, Waldhausl W, Roden M (2001) Lipid-dependent control of hepatic glycogen stores in healthy humans. Diabetologia 44: 48–54
Boden G, Chen X, Rosner J, Barton M (1995) Effects of a 48-h fat infusion on insulin secretion and glucose utilization. Diabetes 44: 1239–1242
Carpentier A, Mittelman SD, Bergman RN, Giacca A, Lewis GF (2000) Prolonged elevation of plasma free fatty acids impairs pancreatic beta-cell function in obese nondiabetic humans but not in individuals with type 2 diabetes. Diabetes 49: 399–408
Krssak M, Falk Petersen K, Dresner A, DiPietro L, Vogel SM, Rothman DL, Roden M, Shulman GI (1999) Intramyocellular lipid concentrations are correlated with insulin sensitivity in humans: a 1H NMR spectroscopy study. Diabetologia 42: 113–116
Anderwald C, Bernroider E, Krssak M, Stingl H, Brehm A, Bischof MG, Nowotny P, Roden M, Waldhausl W (2002) Effects of insulin treatment in type 2 diabetic patients on intracellular lipid content in liver and skeletal muscle. Diabetes 51: 3025–3032
Ratheiser K, Schneeweiss B, Waldhausl W, Fasching P, Korn A, Nowotny P, Rohac M, Wolf HP (1991) Inhibition by etomoxir of carnitine palmitoyltransferase I reduces hepatic glucose production and plasma lipids in noninsulin-dependent diabetes mellitus. Metabolism 40: 1185–1190
Dobbins RL, Szczepaniak LS, Bentley B, Esser V, Myhill J, McGarry JD (2001) Prolonged inhibition of muscle carnitine palmitoyltransferase-1 promotes intramyocellular lipid accumulation and insulin resistance in rats. Diabetes 50: 123–130
Fulcher GR, Catalano C, Walker M, Farrer M, Thow J, Whately-Smith CR, Alberti KG (1992) A double blind study of the effect of acipimox on serum lipids, blood glucose control and insulin action in non-obese patients with type 2 diabetes mellitus. Diabet Med 9: 908–914
Saloranta C, Groop L, Ekstrand A, Franssila-Kallunki A, Eriksson J, Taskinen MR (1993) Different acute and chronic effects of acipimox treatment on glucose and lipid metabolism in patients with type 2 diabetes. Diabet Med 10: 950–957
Worm D, Henriksen JE, Vaag A, Thye-Ronn P, Melander A, Beck-Nielsen H (1994) Pronounced blood glucose-lowering effect of the antilipolytic drug acipimox in noninsulin-dependent diabetes mellitus patients during a 3-day intensified treatment period. J Clin Endocrinol Metab 78: 717–721
Winder WW, Hardie DG (1999) AMP-activated protein kinase, a metabolic master switch: possible roles in type 2 diabetes. Am J Physiol 277: E1–10
Bavenholm PN, Pigon J, Saha AK, Ruderman NB, Efendic S (2000) Fatty acid oxidation and the regulation of malonyl-CoA in human muscle. Diabetes 49: 1078–1083
Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, Wu M, Ventre J, Doebber T, Fujii N, Musi N, Hirshman MF, Goodyear LJ, Moller DE (2001) Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest 108: 1167–1174
Musi N, Hirshman MF, Nygren J, Svanfeldt M, Bavenholm P, Rooyackers O, Zhou G, Williamson JM, Ljunqvist O, Efendic S, Moller DE, Thorell A, Goodyear LJ (2002) Metformin increases AMP-activated protein kinase activity in skeletal muscle of subjects with type 2 diabetes. Diabetes 51: 2074–2081
Hawley SA, Gadalla AE, Olsen GS, Hardie DG (2002) The antidiabetic drug metformin activates the AMP-activated protein kinase cascade via an adenine nucleotideindependent mechanism. Diabetes 51: 2420–2425
Bergeron R, Russell RR, Young LL, Ren JM, Marcucci M, Lee A, Shulman GI (1999) Effect of AMPK activation on muscle glucose metabolism in conscious rats. Am J Physiol 276: E938–944
Iglesias MA, Ye JM, Frangioudakis G, Saha AK, Tomas E, Ruderman NB, Cooney GJ, Kraegen EW (2002) AICAR administration causes an apparent enhancement of muscle and liver insulin action in insulin-resistant highfat-fed rats. Diabetes 51: 2886–2894
Fajas L, Auboeuf D, Raspe E, Schoonjans K, Lefebvre AM, Saladin R, Najib J, Laville M, Fruchart JC, Deeb S, Vidal-Puig A, Flier J, Briggs MR, Staels B, Vidal H, Auwerx J (1997) The organization, promoter analysis, and expression of the human PPAR gamma gene. J Biol Chem 272: 18779–18789
Mudaliar S, Henry RR (2001) New oral therapies for type 2 diabetes mellitus: The glitazones or insulin sensitizers. Annu Rev Med 52: 239–257
Lehmann JM, Moore LB, Smith-Oliver TA, Wilkison WO, Willson TM, Kliewer SA (1995) An antidiabetic thiazolidinedione is a high affinity ligand for peroxisome proliferator-activated receptor gamma (PPAR gamma). J Biol Chem 270: 12953–12956
Willson TM, Brown PJ, Sternbach DD, Henke BR (2000) The PPARs: from orphan receptors to drug discovery. J Med Chem 43: 527–550
Fajas L, Fruchart JC, Auwerx J (1998) Transcriptional control of adipogenesis. Curr Opin Cell Biol 10: 165–173
Barak Y, Nelson MC, Ong ES, Jones YZ, Ruiz-Lozano P, Chien KR, Koder A, Evans RM (1999) PPAR gamma is required for placental, cardiac, and adipose tissue development. Mol Cell 4: 585–595
Kubota N, Terauchi Y, Miki H, Tamemoto H, Yamauchi T, Komeda K, Satoh S, Nakano R, Ishii C, Sugiyama T, Eto K, Tsubamoto Y, Okuno A, Murakami K, Sekihara H, Hasegawa G, Naito M, Toyoshima Y, Tanaka S, Shiota K, Kitamura T, Fujita T, Ezaki O, Aizawa S, Kadowaki T, et al (1999) PPAR gamma mediates high-fat diet-induced adipocyte hypertrophy and insulin resistance. Mol Cell 4: 597–609
Rosen ED, Sarraf P, Troy AE, Bradwin G, Moore K, Milstone DS, Spiegelman BM, Mortensen RM (1999) PPAR gamma is required for the differentiation of adipose tissue in vivo and in vitro. Mol Cell 4: 611–617
Deeb SS, Fajas L, Nemoto M, Pihlajamaki J, Mykkanen L, Kuusisto J, Laakso M, Fujimoto W, Auwerx J (1998) A Prol 2Ala substitution in PPARgamma2 associated with decreased receptor activity, lower body mass index and improved insulin sensitivity. Nat Genet 20: 284–287
Okuno A, Tamemoto H, Tobe K, Ueki K, Mori Y, Iwamoto K, Umesono K, Akanuma Y, Fujiwara T, Horikoshi H, Yazaki Y, Kadowaki T (1998) Troglitazone increases the number of small adipocytes without the change of white adipose tissue mass in obese Zucker rats. J Clin Invest 101: 1354–1361
Olefsky JM (1976) The effects of spontaneous obesity on insulin binding, glucose transport, and glucose oxidation of isolated rat adipocytes. J Clin Invest 57: 842–851
Berger J, Moller DE (2002) The mechanisms of action of PPARs. Annu Rev Med 53: 409–435
Martin G, Schoonjans K, Lefebvre AM, Staels B, Auwerx J (1997) Coordinate regulation of the expression of the fatty acid transport protein and acyl-CoA synthetase genes by PPARalpha and PPARgamma activators. J Biol Chem 272: 28210–28217
Oakes ND, Camilleri S, Furler SM, Chisholm DJ, Kraegen EW (1997) The insulin sensitizer, BRL 49653, reduces systemic fatty acid supply and utilization and tissue lipid availability in the rat. Metabolism 46: 935–942
Schoonjans K, Auwerx J (2000) Thiazolidinediones: an update. Lancet 355: 1008–1010
Furnsinn C, Waldhausl W (2002) Thiazolidinediones: metabolic actions in vitro. Diabetologia 45: 1211–1223
Furnsinn C, Brunmair B, Meyer M, Neschen S, Furtmuller R, Roden M, Kuhnle HF, Nowotny P, Schneider B, Waldhausl W (1999) Chronic and acute effects of thiazolidinediones BM13.1258 and BM15.2054 on rat skeletal muscle glucose metabolism. Br J Pharmacol 128: 1141–1148
Park KS, Ciaraldi TP, Abrams-Carter L, Mudaliar S, Nikoulina SE, Henry RR (1998) Troglitazone regulation of glucose metabolism in human skeletal muscle cultures from obese type II diabetic subjects. J Clin Endocrinol Metab 83: 1636–1643
Preininger K, Stingl H, Englisch R, Furnsinn C, Graf J, Waldhausl W, Roden M (1999) Acute troglitazone action in isolated perfused rat liver. Br J Pharmacol 126: 372–378
Carey DG, Cowin GJ, Galloway GJ, Jones NP, Richards JC, Biswas N, Doddrell DM (2002) Effect of rosiglitazone on insulin sensitivity and body composition in type 2 diabetic patients [corrected] Obes Res 10: 1008–1015
Mayerson AB, Hundal RS, Dufour S, Lebon V, Befroy D, Cline GW, Enocksson S, Inzucchi SE, Shulman GI, Petersen KF (2002) The effects of rosiglitazone on insulin sensitivity, lipolysis, and hepatic and skeletal muscle triglyceride content in patients with type 2 diabetes. Diabetes 51: 797–802
Hallsten K, Virtanen KA, Lonnqvist F, Sipila H, Oksanen A, Viljanen T, Ronnemaa T, Viikari J, Knuuti J, Nuutila P (2002) Rosiglitazone but not metformin enhances insulin- and exercise-stimulated skeletal muscle glucose uptake in patients with newly diagnosed type 2 diabetes. Diabetes 51: 3479–3485
Phillips LS, Grunberger G, Miller E, Patwardhan R, Rappaport EB, Salzman A (2001) Once-and twice-daily dosing with rosiglitazone improves glycemic control in patients with type 2 diabetes. Diabetes Care 24: 308–315
Einhorn D, Rendell M, Rosenzweig J, Egan JW, Mathisen AL, Schneider RL (2000) Pioglitazone hydrochloride in combination with metformin in the treatment of type 2 diabetes mellitus: a randomized, placebo-controlled study. The Pioglitazone 027 Study Group. Clin Ther 22: 1395–1409
Freed MI, Ratner R, Marcovina SM, Kreider MM, Biswas N, Cohen BR, Brunzell JD (2002) Effects of rosiglitazone alone and in combination with atorvastatin on the metabolic abnormalities in type 2 diabetes mellitus. Am J Cardiol 90: 947–952
Rubins HB, Robins SJ (2000) Conclusion from the VAHIT study. Am J Cardiol 86: 543–544
Guerre-Millo M, Gervois P, Raspe E, Madsen L, Poulain P, Derudas B, Herbert JM, Winegar DA, Willson TM, Fruchart JC, Berge RK, Staels B (2000) Peroxisome proliferator-activated receptor alpha activators improve insulin sensitivity and reduce adiposity. J Biol Chem 275: 16638–16642
Braissant O, Foufelle F, Scotto C, Dauca M, Wahli W (1996) Differential expression of peroxisome proliferator-activated receptors (PPARs): tissue distribution of PPAR-alpha,-beta, and-gamma in the adult rat. Endocrinology 137: 354–366
Zhou YT, Shimabukuro M, Wang MY, Lee Y, Higa M, Milburn JL, Newgard CB, Unger RH (1998) Role of peroxisome proliferator-activated receptor alpha in disease of pancreatic beta cells. Proc Natl Acad Sci USA 95: 8898–8903
Kersten S, Seydoux J, Peters JM, Gonzalez FJ, Desvergne B, Wahli W (1999) Peroxisome proliferator-activated receptor alpha mediates the adaptive response to fasting. J Clin Invest 103: 1489–1498
Guerre-Millo M, Rouault C, Poulain P, Andre J, Poitout V, Peters JM, Gonzalez FJ, Fruchart JC, Reach G, Staels B (2001) PPAR-alpha-null mice are protected from high-fat diet-induced insulin resistance. Diabetes 50: 2809–2814
Ye JM, Doyle PJ, Iglesias MA, Watson DG, Cooney GJ, Kraegen EW (2001) Peroxisome proliferator-activated receptor (PPAR)-alpha activation lowers muscle lipids and improves insulin sensitivity in high fat-fed rats: comparison with PPAR-gamma activation. Diabetes 50: 411–417
Lohray BB, Lohray VB, Bajji AC, Kalchar S, Poondra RR, Padakanti S, Chakrabarti R, Vikramadithyan RK, Misra P, Juluri S, Mamidi NV, Rajagopalan R (2001) (−)-3-[4-[2-(Phenoxazin-10-yl)ethoxy]phenyl]-2-ethoxypropanoic acid [(−)DRF 2725]: a dual PPAR agonist with potent antihyperglycemic and lipid modulating activity. J Med Chem 44: 2675–2678
Yajima K, Hirose H, Fujita H, Seto Y, Ukeda K, Miyashita K, Kawai T, Yamamoto Y, Ogawa T, Yamada T, Saruta T (2003) Combination therapy with PPAR gamma and PPAR alpha agonists increases glucose-stimulated insulin secretion in db/db mice. Am J Physiol Endocrinol Metab 284: E966–971
Murakami K, Tobe K, Ide T, Mochizuki T, Ohashi M, Akanuma Y, Yazaki Y, Kadowaki T (1998) A novel insulin sensitizer acts as a coligand for peroxisome proliferatoractivated receptor-alpha (PPAR-alpha) and PPAR-gamma: effect of PPAR-alpha activation on abnormal lipid metabolism in liver of Zucker fatty rats. Diabetes 47: 1841–1847
Etgen GJ, Oldham BA, Johnson WT, Broderick CL, Montrose CR, Brozinick JT, Misener EA, Bean JS, Bensch WR, Brooks DA, Shuker AJ, Rito CJ, McCarthy JR, Ardecky RJ, Tyhonas JS, Dana SL, Bilakovics JM, Paterniti JR, Jr Ogilvie KM, Liu S, Kauffman RF (2002) A tailored therapy for the metabolic syndrome: the dual peroxisome proliferator-activated receptor-alpha/gamma agonist LY465608 ameliorates insulin resistance and diabetic hyperglycemia while improving cardiovascular risk factors in preclinical models. Diabetes 51: 1083–1087
Ljung B, Bamberg K, Dahllof B, Kjellstedt A, Oakes ND, Ostling J, Svensson L, Camejo G (2002) AZ 242, a novel PPARalpha/gamma agonist with beneficial effects on insulin resistance and carbohydrate and lipid metabolism in ob/ob mice and obese Zucker rats. J Lipid Res 43: 1855–1863
Ye JM, Iglesias MA, Watson DG, Ellis B, Wood L, Jensen PB, Sorensen RV, Larsen PJ, Cooney GJ, Wassermann K, Kraegen EW (2003) PPARalpha/gamma ragaglitazar eliminates fatty liver and enhances insulin action in fatfed rats in the absence of hepatomegaly. Am J Physiol Endocrinol Metab 284: E531–540
Brand CL, Sturis J, Gotfredsen CF, Flecknerr J, Fledelius C, Hansen BF, Andersen B, Ye JM, Sauerberg P, Wassermann K (2003) Dual PPARalpha/gamma activation provides enhanced improvement of insulin sensitivity and glycemic control in ZDF rats. Am J Physiol Endocrinol Metab 284: E841–854
Skrumsager BK, Nielsen KK, Muller M, Pabst G, Drake PG, Edsberg B (2003) Ragaglitazar: the pharmacokinetics, pharmacodynamics, and tolerability of a novel dual PPAR alpha and gamma agonist in healthy subjects and patients with type 2 diabetes. J Clin Pharmacol 43: 1244–1256
Rui L, Aguirre V, Kim JK, Shulman GI, Lee A, Corbould A, Dunaif A, White MF (2001) Insulin/IGF-1 and TNF-alpha stimulate phosphorylation of IRS-1 at inhibitory Ser307 via distinct pathways. J Clin Invest 107: 181–189
Engelman JA, Berg AH, Lewis RY, Lisanti MP, Scherer PE (2000) Tumor necrosis factor alpha-mediated insulin resistance, but not dedifferentiation, is abrogated by MEK 1/2 inhibitors in 3T3-L1 adipocytes. Mol Endocrinol 14: 1557–1569
Segal KR, Landt M, Klein S (1996) Relationship between insulin sensitivity and plasma leptin concentration in lean and obese men. Diabetes 45: 988–991
Wang J, Obici S, Morgan K, Barzilai N, Feng Z, Rossetti L (2001) Overfeeding rapidly induces leptin and insulin resistance. Diabetes 50: 2786–2791
Roden M, Ludwig C, Nowotny P, Schneider B, Clodi M, Vierhapper H, Roden A, Waldhausl W (2000) Relative hypoleptinemia in patients with type 1 and type 2 diabetes mellitus. Int J Obes Relat Metab Disord 24: 976–981
Anderwald C, Muller G, Koca G, Furnsinn C, Waldhausl W, Roden M (2002) Short-term leptin-dependent inhibition of hepatic gluconeogenesis is mediated by insulin receptor substrate-2. Mol Endocrinol 16: 1612–1628
Roden M, Anderwald C, Furnsinn C, Waldhausl W, Lohninger A (2000) Effects of short-term leptin exposure on triglyceride deposition in rat liver. Hepatology 32: 1045–1049
Petersen KF, Oral EA, Dufour S, Befroy D, Ariyan C, Yu C, Cline GW, DePaoli AM, Taylor SI, Gorden P, Shulman GI (2002) Leptin reverses insulin resistance and hepatic steatosis in patients with severe lipodystrophy. J Clin Invest 109: 1345–1350
Farooqi IS, Jebb SA, Langmack G, Lawrence E, Cheetham CH, Prentice AM, Hughes IA, McCamish MA, O’Rahilly S (1999) Effects of recombinant leptin therapy in a child with congenital leptin deficiency. N Engl J Med 341: 879–884
Fruebis J, Tsao TS, Javorschi S, Ebbets-Reed D, Erickson MR, Yen FT, Bihain BE, Lodish HF (2001) Proteolytic cleavage product of 30-kDa adipocyte complement-related protein increases fatty acid oxidation in muscle and causes weight loss in mice. Proc Natl Acad Sci USA 98: 2005–2010
Berg AH, Combs TP, Du X, Brownlee M, Scherer PE (2001) The adipocyte-secreted protein Acrp30 enhances hepatic insulin action. Nat Med 7: 947–953
Yamauchi T, Kamon J, Waki H, Terauchi Y, Kubota N, Hara K, Mori Y, Ide T, Murakami K, Tsuboyama-Kasaoka N, Ezaki O, Akanuma Y, Gavrilova O, Vinson C, Reitman ML, Kagechika H, Shudo K, Yoda M, Nakano Y, Tobe K, Nagai R, Kimura S, Tomita M, Froguel P, Kadowaki T (2001) The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med 7: 941–946
Yang WS, Lee WJ, Funahashi T, Tanaka S, Matsuzawa Y, Chao CL, Chen CL, Tai TY, Chuang LM (2001) Weight reduction increases plasma levels of an adipose-derived anti-inflammatory protein, adiponectin. J Clin Endocrinol Metab 86: 3815–3819
Yang WS, Jeng CY, Wu TJ, Tanaka S, Funahashi T, Matsuzawa Y, Wang JP, Chen CL, Tai TY, Chuang LM (2002) Synthetic peroxisome proliferator-activated receptor-gamma agonist, rosiglitazone, increases plasma levels of adiponectin in type 2 diabetic patients. Diabetes Care 25: 376–380
Ye JM, Frangioudakis G, Iglesias MA, Furler SM, Ellis B, Dzamko N, Cooney GJ, Kraegen EW (2002) Prior thiazolidinedione treatment preserves insulin sensitivity in normal rats during acute fatty acid elevation: role of the liver. Endocrinology 143: 4527–4535
Hirose H, Kawai T, Yamamoto Y, Taniyama M, Tomita M, Matsubara K, Okazaki Y, Ishii T, Oguma Y, Takei I, Saruta T (2002) Effects of pioglitazone on metabolic parameters, body fat distribution and serum adiponectin levels in Japanese male patients with type 2 diabetes. Metabolism 51: 314–317
Hundal RS, Petersen KF, Mayerson AB, Randhawa PS, Inzucchi S, Shoelson SE, Shulman GI (2002) Mechanism by which high-dose aspirin improves glucose metabolism in type 2 diabetes. J Clin Invest 109: 1321–1326
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Stingl, H., Roden, M. Zukünftige Angriffspunkte für die Therapie des Typ-2-Diabetes. Wien Klin Wochenschr 116, 217–229 (2004). https://doi.org/10.1007/BF03041051
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF03041051