Abstract
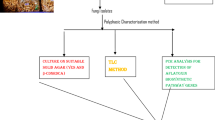
Aspergillus flavus, A parasiticus, A nomius, A tamarii andA pseudotamarii are important microorganisms capable of producing aflatoxins and further mycotoxins. Aflatoxigenic Aspergillus species are morphologically similar species belonging to the Aspergillus section Flavi. The aflatoxigenic fungal strains were isolated from foods (cereals, pulses, oilseeds, dried fruit, spices), soil, air and water. Mycological analyses are based on valid standards and recommendations of the International Commission for Food Mycology (ICFM). The identification of isolated aflatoxigenic fungi in foodstuffs and feedstuffs can be proved by using classical mycological cultivation methods, diagnostic nutrient media, chemotaxonomy and molecular biological methods (PCR). The system approach to the identification of aflatoxigenic fungi combines these four methods. Thirty strains of the aflatoxigenic fungi were tested.
Similar content being viewed by others
References
Wild CP, Hall AJ (1997) Epidemiology of Mycotoxins — Related Disease In: Howard DH and Miller JD (Eds) The Mycota — Human and Animal Relationships VI. Berlin Heidelberg New York, Springer Verlag, p. 213–227.
Zeng-mei S, Zu-tong Q (1991) A new aflatoxin producing species of section Flavi of Aspergillus. Acta Mycologica Sinica, 10: 22–26
Saito M, Machida S (1999) A rapid identification method for aflatoxin-producing strains ofA flavus andA parasiticus by ammonia vapor. Mycosci. 40: 205–208
Ito Y, Peterson SW, Wicklow DT, Goto T (2001)A pseudotamarii, a new aflatoxin producing species in Aspergillus section Flavi. Mycol. Res., 105, 2: 233–239
Ostry V, Ruprich J, Skarkova J (1999) The estimaton of dietary exposure of aflatoxins B1 from toxigenic strains ofA flavus, A parasiticus andA nomius by means of the determination aflatoxin M1 in human urine. In: Tuijtelaars ACJ, Samson RA, Rombouts, FM, Notermans S (Eds), Food microbiology and food safety into the next millenium, Ponsen & Looyen Wageningen, pp. 140–144
Sweeney MJ, Dobson ADW (1998) Mycotoxin production by Aspergillus, Fusarium and Penicillium species. Intern. J. Food Microbiol. 43 (3): 141–158
Pitt JI, Hocking AD (1997) Fungi and Food spoilage. London-New York-Tokyo-Melbourne-Madras, Blackie Academic & Professional, pp. 593
Samson RA, Hoekstra ES (1988) Introduction to food-borne fungi, Baarn, CBS: 299.
Reiss J (1998) Schimmelpilze. Berlin-Heidelberg, Springer Verlag, pp. 308
Reshma SV, Ahmad R (1998) Natural incidence of aflatoxins in parboiled rice during various stages of processing. J. Food Sci. Technol. 35 (5): 451–454
Pitt JI, Hocking AD, Samson RA, King AD (1992) Recommended methods for mycological examination of foods In: Samson RAet al. (Eds) Modern methods in food mycology, Elsevier Amsterdam-London-New York-Tokyo, pp. 365–368
Färber P, Geisen R, Holzapfel WH (1997) Detection of aflatoxigenic fungi in figs by a PCR reaction. Intern. J. Food Microbiol., 36: 215–220
Shapira R, Paster N, Eyal O, Menesherov M, Mett A, Salomon R (1996) Detection of aflatoxigenic molds in grain by PCR. Appl. Environ. Microbiol. 62: 3270–3273
Ostry V, Ruprich J (1994) Detection of fungi (Aspergillus, Penicillium spp.) by latex agglutination tests in the Czech Republic. In: Abstrakt book of 7th International Congres of Mycology Division, Prague, p. 459
Kubatova A, Vanova M, Prasil K and Fassatiova O (1999) Microfungi contaminating foods with low water content. Novit. Bot. Univ. Carol. 13: 13–25
Pitt JI, Hocking AD and Glenn DR (1983) An improved medium for the detection ofAspergillus flavus andA parasiticus. J. Appl. Bacteriol. 54: 109–114.
Abarca ML, Bragulat MR, Castella G, Cabanes FJ (1994) Mycoflora and aflatoxin-producing strains in animal mixed feeds. J. Food Protection 57, (3): 256–258.
Paterson RRM (1985) Standardized one- and two-dimensional thin-layer chromatographic methods for the identification of secondary metabolites in Penicillium and other fungi. J. Chromatogr. 368: 249–264
Tran-Dinh N, Kumar S, Pitt JI and Carter DA (2000) Analysis of the molecular and evolutionary basis of toxigenicity and nontoxinogenicity inA flavus andA parasiticus. In: Samson RA and Pitt JI (Eds) Integration of modern taxonomic methods for Penicillium and Aspergillus classification. Harwood academic publishers, p. 435–446
Peterson SW, Horn BW, Ito Y and Goto T (2000) Genetic variation and aflatoxin production inAspergillus tamarii andA caelatus. In: Samson RA and Pitt JI (Eds) Alena Integration of modern taxonomic methods for Penicillium and Aspergillus classification. Harwood academic publishers, p. 447–458
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ostry, V., Ruprich, J., Skarkova, J. et al. The system approach to the identification of aflatoxigenic fungi in foodstuffs and feedstuffs. Mycotox Res 17 (Suppl 2), 178–182 (2001). https://doi.org/10.1007/BF03036431
Issue Date:
DOI: https://doi.org/10.1007/BF03036431