Abstract
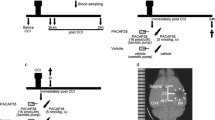
Severalin vitro andin vivo experiments have demonstrated the neuroprotective effects of pituitary adenylate cyclase activating polypeptide (PACAP) in focal cerebral ischemia, Parkinson’s disease and traumatic brain injury (TBI). The aim of the present study was to analyze the effect of PACAP administration on diffuse axonal injury (DAI), an important contributor to morbidity and mortality associated with TBI, in a central fluid percussion (CFP) model of TBI. Rats were subjected to moderate (2 Atm) CFP injury. Thirty min after injury, 100 μg PACAP was administered intracerebroventricularly. DAI was assessed by immunohistochemical detection of β-amyloid precursor protein, indicating impaired axoplasmic transport, and RMO-14 antibody, representing foci of cytoskeletal alterations (neurofilament compaction), both considered classical markers of axonal damage. Analysis of damaged, immunoreactive axonal profiles revealed significant axonal protection in the PACAP-treated versus vehicletreated animals in the corticospinal tract, as far as traumatically induced disturbance of axoplasmic transport and cytoskeletal alteration were considered. Similarly to our former observations in an impact acceleration model of diffuse TBI, the present study demonstrated that PACAP also inhibits DAI in the CFP injury model. The finding indicates that PACAP and derivates can be considered potential candidates for further experimental studies, or purportedly for clinical trials in the therapy of TBI.
Similar content being viewed by others
Abbreviations
- APP:
-
Amyloid precursor protein
- CFP:
-
Central fluid percussion
- CSpT:
-
Corticospinal tract
- DAI:
-
Diffuse axonal injury; icv, intracerebroventricularly
- MLF:
-
Medial longitudinal fascicle
- PACAP:
-
Pituitary adenylate cyclase activating polypeptide
- TBI:
-
Traumatic brain injury
References
Atlasz T, N Babai, P Kiss, D Reglodi, A Tamas, K Szabadfi, G Toth, O Hegyi, A Lubics and R Gabriel (2007) Pituitary adenylate cyclase activating polypeptide is protective in bilateral carotid occlusion-induced retinal lesion in rats.Gen. Comp. Endocrinol. 153, 108–114.
Aubert N, A Falluel-Morel, D Vaudry, X Xifro, J Rodriguez-Alvarez, C Fisch, S de Jouffrey, JF Lebigot, A Fournier, H Vaudry and BJ Gonzalez (2006) PACAP and C2-ceramide generate different AP-1 complexes through a MAP-kinase- dependent pathway: involvement of c-fos in PACAP-induced Bcl-2 expression.J. Neurochem. 99, 1237–1250.
Babai N, T Atlasz, A Tamás, D Reglodi, P Kiss and R Gábriel (2005) Degree of damage compensation by various PACAP treatments in monosodium glutamate-induced retina degeneration.Neurotox. Res. 8, 227–233.
Beal MF, T Palomo, RM Kostrzewa and T Archer (2000) Neuroprotective and neurorestorative strategies for neuronal injury.Neurotox. Res. 2, 71–84.
Blumbergs PC, G Scott, J Manavis, H Wainwright, DA Simpson and AJ McLean (1994) Staining of amyloid precursor protein to study axonal damage in mild head injury.Lancet 344, 1055–1056.
Bramlett HM and WD Dietrich (2004) Pathophysiology of cerebral ischemia and brain trauma: similarities and differences.J. Cereb. Blood Flow Metab. 24, 133–150.
Bramlett HM, S Kraydieh, EJ Green and WD Dietrich (1997) Temporal and regional patterns of axonal damage following traumatic brain injury: a β-amyloid precursor protein immunocytochemical study in rats.J. Neuropathol. Exp. Neurol. 56, 1132–1141.
Brenneman DE (2007) Neuroprotection: a comparative view of vasoactive intestinal peptide and pituitary adenylate cyclase activating polypeptide.Peptides 28, 1720–1726.
Buki A, H Koizumi and JT Povlishock (1999a) Moderate post-traumatic hypothermia decreases early calpain-mediated proteolysis and concomitant cytoskeletal compromise in traumatic axonal injury.Exp. Neurol. 159, 319–328.
Buki A, DO Okonkwo and JT Povlishock (1999b) Postinjury cyclosporin A administration limits axonal damage and disconnection in traumatic brain injury.J. Neurotrauma 16, 511–521.
Buki A, R Siman, JQ Trojanowski and JT Povlishock (1999c) The role of calpain-mediated spectrin proteolysis in traumatically induced axonal injury.J. Neuropathol. Exp. Neurol. 58, 365–375.
Buki A, DO Okonkwo, KK Wang and JT Povlishock (2000) Cytochromec release and caspase activation in traumatic axonal injury.J. Neurosci. 20, 2825–2834.
Buki A, O Farkas, T Doczi and JT Povlishock (2003) Preinjury administration of the calpain inhibitor MDL-28170 attenuates traumatically induced axonal injury.J. Neurotrauma 20, 261–268.
Cernak I, S Chapman, G Hamlin and R Vink (2002) Temporal characterisation of pro- and anti-apoptotic mechanisms following diffuse traumatic brain injury in rats.J. Clin. Neurosci. 9, 565–572.
Chen WH and SF Tzeng (2005) Pituitary adenylate cyclase activating polypeptide prevents cell death in the spinal cord with traumatic injury.Neurosci. Lett. 384, 117–121.
Dejda A, P Sokolowska and JZ Nowak (2005) Neuroprotective potential of three neuropeptides PACAP, VIP and PHI.Pharmacol. Rep. 57, 307–320.
Delgado M, J Leceta and D Ganea (2003) Vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide inhibit the production of inflammatory mediators by activated microglia.J. Leukoc. Biol. 73, 155–164.
De Yebenes JG and MA Mena (2000) Neurotrophic factors in neurodegenerative disorders: model of Parkinson’s disease.Neurotox. Res. 2, 115–137.
Dixon CE, BG Lyeth, JT Povlishock, RL Findling, RJ Hamm, A Marmarou, HF Young and RL Hayes (1987) A fluid percussion model of experimental brain injury in the rat.J. Neurosurg. 67, 110–119.
Doberer D, M Gschwandtner, W Mosgoeller, C Bieglmayer, H Heinzl and V Petkov (2007) Pulmonary and systemic effects of inhaled PACAP38 in healthy male subjects.Eur. J. Clin. Invest. 37, 665–672.
Dohi K, H Mizushima, S Nakajo, H Ohtaki, S Matsunaga, T Aruga and S Shioda (2002) Pituitary adenylate cyclase- activating polypeptide (PACAP) prevents hippocampal neurons from apoptosis by inhibiting JNK/SAPK and p38 signal transduction pathways.Regul. Pept. 109, 83–88.
Farkas O, A Tamas, A Zsombok, D Reglodi, J Pal, A Buki, I Lengvari, JT Povlishock and T Doczi (2004) Effects of pituitary adenylate cyclase activating polypeptide in a rat model of traumatic brain injury.Regul. Pept. 123, 69–75.
Farkas O, B Polgar, J Szekeres-Bartho, T Doczi, JT Povlishock and A Buki (2005) Spectrin breakdown products in the cerebrospinal fluid in severe head injury — preliminary observations.Acta Neurochir. (Wien.) 147, 855–861.
Feng SQ, XH Kong, SF Guo, P Wang, L Li, JH Zhong and XF Zhou (2005) Treatment of spinal cord injury with co-grafts of genetically modified Schwann cells and fetal spinal cord cell suspension in the rat.Neurotox. Res. 7, 169–177.
Foda MA and A Marmarou (1994) A new model of diffuse brain injury in rats. Part II. Morphological characterization.J. Neurosurg. 80, 301–313.
Gasz B, B Racz, E Roth, B Borsiczky, A Ferencz, A Tamas, B Cserepes, A Lubics, F Gallyas Jr, G Toth, I Lengvari and D Reglodi (2006) Pituitary adenylate cyclase activating polypeptide protects cardiomyocytes against oxidative stress- induced apoptosis.Peptides 27, 87–94.
Gentleman SM, MJ Nash, CJ Sweeting, DI Graham and GW Roberts (1993) β-Amyloid precursor protein (β-APP) as a marker for axonal injury after head injury.Neurosci. Lett. 160, 139–144.
Gerlach M, KL Double, MB Youdim and P Riederer (2000) Strategies for the protection of dopaminergic neurons against neurotoxicity.Neurotox. Res. 2, 99–114.
Ghirnikar RS, YL Lee and LF Eng (1998) Inflammation in traumatic brain injury: role of cytokines and chemokines.Neurochem. Res. 23, 329–340.
Katahira M, K Yone, Y Arishima, T Nagamine, S Komiya and S Iwata (2003) The neuroprotective efffects of PACAP on spinal cord injury (SCI) in rats.Regul. Pept. 115, 49.
Kim WK, Y Kan, D Ganea, RP Hart, I Gozes and GM Jonakait (2000) Vasoactive intestinal peptide and pituitary adenylyl cyclase-activating polypeptide inhibit tumor necrosis factor- α production in injured spinal cord and in activated microglia via a cAMP-dependent pathway.J. Neurosci. 20, 3622–3630.
Li M, JL Maderdrut, JJ Lertora and V Batuman (2007) Intravenous infusion of pituitary adenylate cyclase activating polypeptide (PACAP) in a patient with multiple myeloma and myeloma kidney injury: a case study.Peptides 28, 1891–1895.
Marmarou CR, SA Walker, CL Davis and JT Povlishock (2005) Quantitative analysis of the relationship between intra- axonal neurofilament compaction and impaired axonal transport following diffuse traumatic brain injury.J. Neurotrauma 22, 1066–1080.
McIntosh TK, L Noble, B Andrews and AI Faden (1987) Traumatic brain injury in the rat: characterization of a midline fluid-percussion model.Cent. Nerv. Syst. Trauma 4, 119–134.
Miyata A, A Arimura, RR Dahl, N Minamino, A Uehara, L Jiang, MD Culler and DH Coy (1989) Isolation of a novel 38 residue-hypothalamic polypeptide which stimulates adenylate cyclase in pituitary cells.Biochem. Biophys. Res. Commun. 164, 567–574.
Okonkwo DO and JT Povlishock (1999) An intrathecal bolus of cyclosporin A before injury preserves mitochondrial integrity and attenuates axonal disruption in traumatic brain injury.J. Cereb. Blood Flow Metab. 19, 443–451.
Okonkwo DO, EH Pettus, J Moroi and JT Povlishock (1998) Alteration of the neurofilament sidearm and its relation to neurofilament compaction occurring with traumatic axonal injury.Brain Res. 784, 1–6.
Povlishock JT and EH Pettus (1996) Traumatically induced axonal damage: evidence for enduring changes in axolemmal permeability with associated cytoskeletal change.Acta Neurochir. Suppl. (Wien.) 66, 81–86.
Povlishock JT, A Marmarou, T McIntosh, JQ Trojanowski and J Moroi (1997) Impact acceleration injury in the rat: evidence for focal axolemmal change and related neurofilament sidearm alteration.J. Neuropathol. Exp. Neurol. 56, 347–359.
Racz B, D Reglodi, P Kiss, N Babai, T Atlasz, R Gabriel, A Lubics, F Gallyas Jr, B Gasz, G Toth, E Roth, O Hegyi, I Lengvari and A Tamas (2006a)In vivo neuroprotection by PACAP in excitotoxic retinal injury: review of effects on retinal morphology and apoptotic signal transduction.Int. J. Neuroprot. Neurodeg. 2, 80–85.
Racz B, F Gallyas Jr, P Kiss, G Toth, O Hegyi, B Gasz, B Borsiczky, A Ferencz, E Roth, A Tamas, I Lengvari, A Lubics and D Reglodi (2006b). The neuroprotective effects of PACAP in monosodium glutamate-induced retinal lesion involves inhibition of proapoptotic signaling pathways.Regul. Pept. 137, 20–26.
Racz B, F Gallyas Jr, P Kiss, A Tamas, A Lubics, I Lengvari, E Roth, G Toth, O Hegyi, ZS Verzar, CS Fabricsek and D Reglodi (2007) Effects of pituitary adenylate cyclase activating polypeptide (PACAP) on the PKA-Bad-14-3-3 signaling pathway in glutamate-induced retinal injury in neonatal rats.Neurotox. Res. 12, 95–104.
Ravni A, S Bourgault, A Lebon, P Chan, L Galas, A Fournier, H Vaudry, B Gonzalez, LE Eiden and D Vaudry (2006) The neurotrophic effects of PACAP in PC 12 cells: control by multiple transduction pathways.J. Neurochem. 98, 321–329.
Ray SK, CE Dixon and NL Banik (2002) Molecular mechanisms in the pathogenesis of traumatic brain injury.Histol. Histopathol. 17, 1137–1152.
Reglodi D, A Tamas, A Somogyvari-Vigh, Z Szanto, E Kertes, L Lenard, A Arimura and I Lengvari (2002) Effects of pretreatment with PACAP on the infarct size and functional outcome in rat permanent focal cerebral ischemia.Peptides 23, 2227–2234.
Reglodi D, A Tamas, A Lubics, L Szalontay and I Lengvari (2004) Morphological and functional effects of PACAP in 6-hydroxydopamine-induced lesion of the substantia nigra in rats.Regul. Pept. 123, 85–94.
Reglodi D, A Tamas, A Lubics, L Szalontay, A Zsombok, O Farkas, A Buki, T Doczi and I Lengvari (2005) Recent results on the neuroprotective effects of PACAP in rat models of focal cerebral ischemia, Parkinson’s disease and traumatic brain injury.Neurotox. Res. 8, 317.
Reglodi D, A Lubics, P Kiss, I Lengvari, B Gaszner, G Toth, O Hegyi and A Tamas (2006) Effect of PACAP in 6-OHDA- induced injury of the substantia nigra in intact young and ovariectomized female rats.Neuropeptides 40, 265–274.
Segura-Aguilar J and RM Kostrzewa (2006) Neurotoxins and neurotoxicity mechanisms. An overview.Neurotox. Res. 10, 263–285.
Shioda S, H Ozawa, K Dohi, H Mizushima, K Matsumoto, S Nakajo, A Takaki, CJ Zhou, Y Nakai and A Arimura (1998) PACAP protects hippocampal neurons against apoptosis: involvement of JNK/SAPK signaling pathway.Ann. NY Acad. Sci. 865, 111–117.
Shioda S, H Ohtaki, T Nakamachi, K Dohi, J Watanabe, S Nakajo, S Arata, S Kitamura, H Okuda, F Takenoya and Y Kitamura (2006) Pleiotropic functions of PACAP in the CNS. Neuroprotection and neurodevelopment.Ann. NY Acad. Sci. 1070, 550–560.
Skoglosa Y, A Lewen, N Takei, L Hillered and D Lindholm (1999) Regulation of pituitary adenylate cyclase activating polypeptide and its receptor type 1 after traumatic brain injury: comparison with brain-derived neurotrophic factor and the induction of neuronal cell death.Neuroscience 90, 235–247.
Somogyvari-Vigh A and D Reglodi (2004) Pituitary adenylate cyclase activating polypeptide: a potential neuroprotective peptide.Review. Curr. Pharm. Des. 10, 2861–2889.
Stone JR, SA Walker and JT Povlishock (1999) The visualization of a new class of traumatically injured axons through the use of a modified method of microwave antigen retrieval.Acta Neuropathol. (Berl.) 97, 335–345.
Stone JR, RH Singleton and JT Povlishock (2000) Antibodies to the C-terminus of the beta-amyloid precursor protein (APP): a site specific marker for the detection of traumatic axonal injury.Brain Res. 871, 288–302.
Stone JR, RH Singleton and JT Povlishock (2001) Intra-axonal neurofilament compaction does not evoke local axonal swelling in all traumatically injured axons.Exp. Neurol. 172, 320–331.
Tabuchi A, K Funaji, J Nakatsubo, M Fukuchi, T Tsuchiya and M Tsuda (2003) Inactivation of aconitase during the apoptosis of mouse cerebellar granule neurons induced by a deprivation of membrane depolarization.J. Neurosci. Res. 71, 504–515.
Tamas A, A Lubics, I Lengvari and D Reglodi (2006a) Protective effects of PACAP in excitotoxic striatal lesion.Ann. NY Acad. Sci. 1070, 570–574.
Tamas A, A Zsombok, O Farkas, D Reglodi, J Pal, A Buki, I Lengvari, JT Povlishock and T Doczi (2006b) Postinjury administration of pituitary adenylate cyclase activating polypeptide (PACAP) attenuates traumatically induced axonal injury in rats.J. Neurotrauma 23, 686–695.
van Landeghem FK, T Weiss, M Oehmichen and A von Deimling (2007) Cellular localization of pituitary adenylate cyclase activating peptide (PACAP) following traumatic brain injury in humans.Acta Neuropathol. 113, 683–693.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kóvesdi, E., Tamás, A., Reglodi, D. et al. Posttraumatic administration of pituitary adenylate cyclase activating polypeptide in central fluid percussion injury in rats. neurotox res 13, 71–78 (2008). https://doi.org/10.1007/BF03033558
Issue Date:
DOI: https://doi.org/10.1007/BF03033558